

噻吩甲酰硫脲衍生物的合成及其抑菌活性
收稿日期: 2016-05-10
修回日期: 2016-06-12
网络出版日期: 2016-07-08
基金资助
公益性行业(农业)科研专项基金(No.201503112)、河南省高等学校重点科研(No.16A210006)资助项目.
Synthesis of Thiophene Formyl Thiourea Derivatives and Fungicidal Activity
Received date: 2016-05-10
Revised date: 2016-06-12
Online published: 2016-07-08
Supported by
Project supported by the Special Fund for Agro-scientific Research in the Public Interest (No.201503112) and the Key Scientific Research Project of Colleges and Universities in Henan Province (No.16A210006).
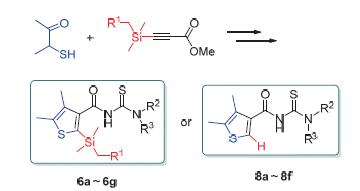
通过3-巯基-2-丁酮与取代硅基丙炔酸甲酯的环合、酯的选择性水解、异硫氰化、胺化等步骤合成了13个未见报道的噻吩甲酰硫脲衍生物.各噻吩甲酰硫脲衍生物的结构经1H NMR、13C NMR和元素分析的确证,其对小麦全蚀病、水稻纹枯病病原菌的抑菌活性经平皿法进行了测试.结果表明,大部分噻吩甲酰硫脲衍生物对小麦全蚀病病原菌具有一定抑制活性,其中1-环丙基-3-(4,5-二甲基噻吩-3-甲酰基)硫脲(8c)抑菌活性表现较为突出,在10 mg/L浓度下其抑菌活性接近对照硅噻菌胺的水平.结构分析表明噻吩-3-甲酰硫脲衍生物噻吩环2位官能团的大小对小麦全蚀病菌抑制活性的影响不明显.
程绎南 , 靳文波 , 谢桂英 , 马艺超 , 赵艳芹 , 李洪连 . 噻吩甲酰硫脲衍生物的合成及其抑菌活性[J]. 有机化学, 2016 , 36(11) : 2683 -2688 . DOI: 10.6023/cjoc201605012
13 New thiophene formyl thiourea derivatives were synthesized via cycloaddition reaction of 3-mercaptobutan-2-one and methyl 3-(substitutedsilyl)propiolate, selective hydrolysis of ester, isothiocyanatation and amination. Their chemical structures were confirmed by 1H NMR, 13C NMR and elemental analysis. The inhibitory activity against Gaeumannomyces graminis var. tritici and Rhizoctonia solani was evaluated in vitro by the plate method. The results indicated that most of thiophene formyl thiourea derivatives showed some inhibitory activity against gaeumannomyces graminis var. tritici. Compound 1-cyclopropyl-3-(4,5-dimethylthiophene-3-carbonyl)thiourea (8c) gave the best performance and its inhibitory activity was close to the control level of silthiopham in the concentration of 10 mg/L. The structure analysis showed that the steric hindrance of functional group at the second position of the thiophene formyl thioureas had no obvious effect on the inhibitory activity against gaeumannomyces graminis var. tritici.

[1] Yan, Z. K.; Song, B. A.; Yang, X.; Hu, D. Y.; Yang, S.; Jin, L. H.; Chen, G. M. Agrochemicals 2008, 47, 706 (in Chinese). (闫志坤, 宋宝安, 杨璇, 胡德禹, 杨松, 金林红, 陈广明, 农药, 2008, 47, 706.)
[2] Ishaaya, I.; Mendelson, Z.; Horowitz, A. R. Phytoparasitica 1993, 21, 199.
[3] Sun, Z. H.; Huang, W.; Gong, Y. Y.; Lan, J.; Liu, X. H.; Weng, J. Q.; Li, Y. S.; Tan, C. X. Chin. J. Org. Chem. 2013, 33, 2612 (in Chinese). (孙召慧, 黄伟, 贡云芸, 蓝健, 刘幸海, 翁建全, 李永曙, 谭成侠, 有机化学, 2013, 33, 2612.)
[4] Miao, H. J.; Zhang, J. W.; Yuan, H. Z.; Li, Y.; Xu, Y.; Li, H.; Yang, X. L.; Ling, Y. Chin. J. Org. Chem. 2012, 32, 915 (in Chinese). (苗宏健, 张继伟, 袁会珠, 李映, 徐焱, 李慧, 杨新玲, 凌云, 有机化学, 2012, 32, 915.)
[5] Saeed, A.; Khan, M. S.; Rafique, H.; Shahid, M.; Iqbal, J. Bioorg. Chem. 2014, 52, 1.
[6] Williams, D. J.; Dimmic, M. W.; Haakenson, W. P. J.; Wideman, A.; Shortt, B. J.; Cheeseright, T.; Crawford, M. J. WO 2009023721, 2009[Chem. Abstr. 2009, 150, 230986].
[7] Dolman, H.; Kuipers, J. EP 234622, 1987[Chem. Abstr. 1988, 109, 110245].
[8] Kim, D. S.; Chun, S. J.; Jeon, J. J.; Lee, S. W.; Joe, G. H. Pest Manage. Sci. 2004, 60, 1007.
[9] Phillion, D.; Wong, S. C.; Shortt, B. US 5486621, 1996[Chem. Abstr. 1996, 124, 253325].
[10] Yoshikawa, Y.; Tomitani, K.; Katsuta, H.; Kawashima, H.; Takahashi, T.; Inami, S.; Yanase, Y.; Takashi, A.; Shimotori, H.; Tomura, N. JP 09301974, 1997[Chem. Abstr. 1997, 128, 22908].
[11] Xie, G. Y.; Jin, W. B.; Zhao, Y. Q.; Cheng, Y. N.; Sun, B. J.; Sun, S. J.; Wang, M. Z.; Wei D. D.; Li, H. L. Chin. J. Org. Chem. 2014, 34, 1124 (in Chinese). (谢桂英, 靳文波, 赵艳芹, 程绎南, 孙炳剑, 孙淑君, 汪梅子, 位丹丹, 李洪连, 有机化学, 2014, 34, 1124.)
[12] Jin, W. B.; Xie, G. Y.; Sun, S. J.; Zhao, Y. Q.; Cheng, Y. N.; Sun, B. J.; Li, H. L. Chin. J. Org. Chem. 2014, 34, 2376 (in Chinese). (靳文波, 谢桂英, 孙淑君, 赵艳芹, 程绎南, 孙炳剑, 李洪连, 有机化学, 2014, 34, 2376.)
[13] Fevig, T. L.; Pillips, W. G.; Lau, P. H. J. Org. Chem. 2001, 66, 2493.
[14] Bures, E.; Spinazze, P. G.; Beese, G.; Hunt, I. R.; Rogers, C.; Keay, B. A. J. Org. Chem. 1997, 62, 8741.
[15] Lu, B.; Falck, J. R. Angew. Chem., Int. Ed. 2008, 47, 7508.
[16] Kuznetsov, A.; Onishi, Y.; Inamoto, Y.; Gevorgyan, V. Org. Lett. 2013, 15, 2498.
[17] Ihara, H.; Koyanagi, M.; Suginome, M. Org. Lett., 2011, 13, 2662.
[18] Cao, H.; Zhan, H. Y.; Wu, J. Y.; Zhong, H. P.; Lin, Y. G.; Zhang, H. Eur. J. Org. Chem. 2012, 2318.
[19] Cao, H.; Jiang, H. F.; Zhou, X. S.; Qi, C. R.; Lin, Y. G.; Wu, J. Y.; Liang, Q. M. Green Chem. 2012, 14, 2710.
[20] Baharfar, R.; Baghbaniana, S. M.; Hossein, N. R.; Bijanzadeh, H. R. Lett. Org. Chem. 2007, 4, 567.
[21] Reddy, L. R.; Saravanan, P.; Corey, E. J. J. Am. Chem. Soc. 2004, 126, 6230.
[22] Downey, C. W.; Craciun, S.; Neferu, A. M.; Vivelo, C. A.; Mueller, C. J.; Southall, B. C.; Corsi, S.; Etchill, E. W.; Sault, R. J. Tetrahedron Lett. 2012, 53, 5763
[23] Bohlmann, F.; Bresinsky, E. Chem. Ber. 1964, 97, 2109.
[24] Sun, B. J.; Yuan, H. X.; Xing, X. P.; Li, H. L. J. Triticeae Crops 2008, 28, 709 (in Chinese). (孙炳剑, 袁虹霞, 邢小萍, 李洪连, 麦类作物学报, 2008, 28, 709.)
/
| 〈 |
|
〉 |