

基于芳基炔醛共轭延伸的1,8-萘酰亚胺的高选择性比色和荧光氰离子探针
收稿日期: 2016-05-16
修回日期: 2016-06-13
网络出版日期: 2016-07-08
基金资助
国家自然科学基金(No.21202099)、上海市科委地方院校能力建设(No.15120503700)资助项目.
A Selective Colorimetric and Fluorescent Diphenylacetylene-Based Naphthalimide for Sensing of Cyanide
Received date: 2016-05-16
Revised date: 2016-06-13
Online published: 2016-07-08
Supported by
Project supported by the National Natural Science Foundation of China (No.21202099),and the Science and Technology Commission of Shanghai Municipality (No.15120503700).
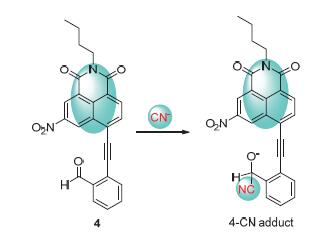
以N-丁基-4-溴-6-硝基-1,8-萘酰亚胺与邻炔基苯甲醛经Sonogashira偶联反应合成了1个高选择性的氰根离子荧光探针4.在乙腈溶液中,探针4对氰根离子具有比色和荧光双重响应.加入氰根离子后,探针4的紫外-可见光谱在540 nm处产生新吸收峰,溶液由无色变成浅紫色,其他阴离子对探针4的紫外-可见光谱几乎无影响.无CN-存在时,探针4的荧光光谱在484 nm附近产生强荧光,加入CN-后,484 nm处的发射带逐渐消失,同时在600 nm附近产生一组新峰,荧光颜色从浅绿色变成浅棕色.这归因于CN-对不饱和醛基进行加成,进而通过共轭炔基影响萘酰亚胺荧光团上的电荷转移.同时,探针4在乙腈/水(体积比9:1)混合体系对阴离子的干扰实验进行了详细的研究.
赵飞飞 , 伍宏伟 , 刘传祥 , 毛海舫 . 基于芳基炔醛共轭延伸的1,8-萘酰亚胺的高选择性比色和荧光氰离子探针[J]. 有机化学, 2016 , 36(11) : 2689 -2694 . DOI: 10.6023/cjoc201605027
A high selective fluorescent probe 4 for cyanides was developed based on the Sonogashira reaction between N-butyl-4-bromo-1,8-naphthalimide and 2-ethynylbenzaldehyde. In CH3CN solution, the probe 4 shows moderate colorimetric and fluorescent response to the cyanides. Upon the addition of TBACN, a new peak at 540 nm appeared in the UV-vis spectra accompanied by an instant colorimetric change from colorless to light violet. No change in the spectral pattern of chemosensor 4 was observed in the presence of other anions. Further, chemosensor 4 showed strong fluorescence with the maximum at 484 nm (λex=390 nm) in a mixture of CH3CN; however, in presence of CN-, a new emission band (λem=600 nm, light brown fluo-rescence) appeared along with a decrease in the emission intensity at 484 nm. Therefore, this process clearly demonstrates that chemosensor 4 can selectively detect cyanide ions by a fluorogenic "on-off" response, which may be attributed to the fact that the electron transfer in 1,8-naphthalimide is affected by the formation of adducts of anion with carbonyl groups. Moreover, the detailed interference experiments of chemosensor 4 in the mixed solvents were also investigated.

[1] Young, C.; Tidwell, L.; Anderson, C. Cyanide:Social, Industrial, and Economic Aspects, The Minerals, Metals & Materials Society, Warrendale, 2001.
[2] Baud, F. J. Hum. Exp. Toxicol. 2007, 26, 191.
[3] Koening, R. Science 2000, 287, 1737.
[4] Vallejos, S.; Estevez, P.; Garcia, F. C.; Serna, F.; Pena, J. L.; Garcia, J. M. Chem. Commun. 2010, 46, 7951.
[5] Way, J. L. Annu. Rev. Pharmacol. 1984, 24, 451.
[6] Zamecnik, J.; Tam, J. J. Anal. Toxicol. 1987, 11, 47.
[7] Anderson, R. A.; Harland, W. A. Med., Sci. Law 1982, 22, 35.
[8] Duke, R. M.; Veale, E. B.; Pfeffer, F. M.; Kruger, P. E.; Gunnlaugsson, T. Chem. Soc. Rev. 2010, 39, 3936.
[9] Zhang, Y. M.; Lin, Q.; Wei, T. B.; Li, Y.; Qin, X. P. Chem. Commun. 2009, 45, 6074.
[10] Du, J.; Hu, M.; Fan, J.; Peng, X. Chem. Soc. Rev. 2012, 41, 4511.
[11] Yang, Y.; Zhao, Q.; Feng, W.; Li, F. Chem. Rev. 2013, 113, 192.
[12] Xu, Z.; Kim, S. K.; Yoon, J. Chem. Soc. Rev. 2010, 39, 1457.
[13] Hu, J. H.; Yan, N. P.; Chen, J. J.; Li, J. B. Chem. J. Chin. Univ. 2013, 34, 1368 (in Chinese). (胡京汉, 颜农平, 陈娟娟, 李建斌, 高等学校化学学报, 2013, 34, 1368.)
[14] Lin, Q.; Liu, X.; Chen, P.; Wei, T. B.; Zhang, Y. M. Prog. Chem. 2013, 25, 2131 (in Chinese). (林奇, 刘昕, 陈佩, 魏太保, 张有明, 化学进展, 2013, 25, 2131.)
[15] Xu, Z.; Chen, X.; Kim, H. N.; Yoon, J. Chem. Soc. Rev. 2010, 39, 127.
[16] Lv, X.; Liu, J.; Liu, Y.; Zhao, Y.; Sun, Y. Q.; Wang, P.; Guo, W. Chem. Commun. 2011, 47, 12843.
[17] Ekmekci, Z.; Yilmaz, M. D.; Akkaya, E. U. Org. Lett. 2008, 10, 461.
[18] Chung, Y. M.; Raman, B.; Kim, D. S.; Ahn, K. H. Chem. Commun. 2006, 42, 186.
[19] Lee, K. S.; Kim, H. J.; Kim, G. H.; Shin, I.; Hong, J. I. Org. Lett. 2008, 10, 49.
[20] Li, H. D.; Li, B.; Jin, L. Y.; Kan, Y. H.; Yin, B. Z. Tetrahedron 2011, 67, 7348.
[21] Jo, J.; Olasz, A.; Chen, C. H.; Lee, D. J. Am. Chem. Soc. 2013, 135, 3620.
[22] Kolosov, D.; Adamovich, V.; Djurovich, P.; Thompson, M. E.; Adachi, C. J. Am. Chem. Soc. 2002, 124, 9945.
[23] Ton, X. A.; Acha, V.; Bonomi, P.; Bui, B. T. S.; Haupt, K. Biosens. Bioelectron. 2015, 64, 359.
[24] Zhu, L.; Yuan, Z.; Simmons, J. T.; Sreenath, K. RSC Adv. 2014, 4, 20398.
[25] Xu, Z. C.; Yoon, J.; Spring, D. R. Chem. Soc. Rev. 2010, 39, 1996.
[26] Sun, J.-F.; Qian, Y. Chin. J. Org. Chem. 2015, 35, 1104 (in Chinese). (孙京府, 钱鹰, 有机化学, 2015, 35, 1104.)
[27] Zhang, C.; Ji, K.; Wang, X.; Wu, H.; Liu, C. Chem. Commun. 2015, 51, 8173.
[28] Zhou, M.; Chen, J.; Liu, C.; Fu, H.; Zheng, N.; Zhang, C.; Chen, Y.; Cheng, J. Chem. Commun. 2014, 50, 14748.
[29] Cai, Y. S.; Guo, Z. Q.; Chen, J. M.; Li, W. L.; Zhong, L. B.; Gao, Y.; Jiang, L.; Chi, L. F.; Tian, H.; Zhu, W. H. J. Am. Chem. Soc. 2016, 138, 2219.
[30] Tomas-Mendivil, E.; Starck, J.; Ortuno, J. C.; Michelet, V. Org. Lett. 2015, 17, 6126.
[31] Scalera, M.; Joyce, A. W. US 2385106, 1945.
[32] Wang, X.; Silverman, R. B. J. Org. Chem. 1998, 63, 7357.
/
| 〈 |
|
〉 |