

石胆酸类似物的设计、合成及其蛋白酪氨酸磷酸酯酶1B抑制活性
收稿日期: 2016-05-12
修回日期: 2016-05-27
网络出版日期: 2016-07-13
基金资助
江苏省自然科学基金青年(No.BK20140425)及南通大学引进人才科研启动费(No.03080694)资助项目.
Design, Synthesis of Lithocholic Acid Mimics and Their Inhibitory Activities against Protein Tyrosine Phosphatase 1B
Received date: 2016-05-12
Revised date: 2016-05-27
Online published: 2016-07-13
Supported by
Project supported by the Science Fund for Young Scholar of Jiangsu Province (No.BK20140425) and the Initiating Fund for Introduced Talents of Nantong University (No.03080694).
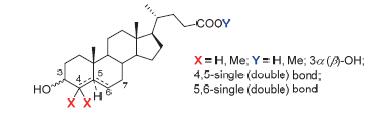
蛋白酪氨酸磷酸酯酶1B(PTP1B)是体内胰岛素信号通路的负调控因子,被视为治疗糖尿病的潜在靶标.甾体类天然产物石胆酸3(LCA)具有较好的PTP1B抑制活性.为了提供石胆酸衍生合成多样性的基本骨架,并探讨甾环上特定位点(3,4,5,6及23位)取代基及其构型与PTP1B抑制活性的关系,设计并合成了一组石胆酸类似物.PTP1B抑制活性测试结果显示,3β-羟基胆烷-4-烯酸(17)和4,4-二甲基-3β-羟基-5-烯-胆烷酸(19)对PTP1B的抑制活性均比石胆酸有所提高,分别达到(8.50±1.21)和(6.27±1.03)μmol·L-1.此外,通过计算机模拟对接阐明了两个化合物与酶的可能结合方式.为进一步研究PTP1B抑制剂提供了新的骨架化合物及有价值的构效关系信息.
何海兵 , 戴红 , 高立信 , 张海军 , 邹政 , 杨帆 , 李佳 , 石玉军 . 石胆酸类似物的设计、合成及其蛋白酪氨酸磷酸酯酶1B抑制活性[J]. 有机化学, 2016 , 36(11) : 2670 -2676 . DOI: 10.6023/cjoc201605018
Protein tyrosine phosphatase-1B (PTP1B), a negative regulatory factor of insulin signaling, is recognized as a potent target for the therapy of diabetes. Aimed to provide new scaffold to the development of PTP1B inhibitors and disclose the relationship between configurations of certain positions (3, 4, 5, 6 and 23-position) on the steroidal skeleton and inhibitory activities against PTP1B, a class of lithocholic acid (LCA) mimics were designed and synthesized. In vitro bioassay against PTP1B showed that 3β-hydroxy-4-ene-cholanic acid (17) and 4,4-dimethyl-3β-hydroxy-5-ene-cholanic acid (19) had activities higher than LCA, reaching (8.50±1.21) and (6.27±1.03) μmol·L-1, respectively. Docking analysis of compounds 17 and 19 illuminated the binding modes to PTP1B. This study provided compounds with new scaffold and valuable structure-activity-relationship (SAR) information for the further study of PTP1B inhibitors.

Key words: PTP1B inhibitor; steroid; lithocholic acid mimics; synthesis
[1] Hu, F. B. Diabetes Care 2011, 34, 1249.
[2] Herman, G. A.; Stevens, C.; Van Dyck, K.; Bergman, A.; Yi, B.; De Smet, M.; Snyder, K.; Hilliard, D.; Tanen, M.; Tanaka, W.; Wang, A. Q.; Zeng, W.; Musson, D.; Winchell, G.; Davies, M. J.; Ramael, S.; Gottesdiener, K. M.; Wagner, J. A. Clin. Pharmacol. Ther. 2005, 78, 675.
[3] Augeri, D. J.; Robl, J. A.; Betebenner, D. A.; Magnin, D. R.; Khanna, A.; Robertson, J. G.; Wang, A.; Simpkins, L. M.; Taunk, P.; Huang, Q.; Han, S.-P.; Abboa-Offei, B.; Cap, M.; Xin, L.; Tao, L.; Tozzo, E.; Welzel, G. E.; Egan, D. M.; Marcinkeviciene, J.; Chang, S. Y.; Biller, S. A.; Kirby, M. S.; Parker, R. A.; Hamann, L. G. J. Med. Chem. 2005, 48, 5025.
[4] Degn, K. B.; Juhl, C. B.; Sturis, J.; Jakobsen, G.; Brock, B.; Chandramouli, V.; Rungby, J.; Landau, B. R.; Schmitz, O. Diabetes 2004, 53, 1187.
[5] Nunez, D. J.; Bush, M. A.; Collins, D. A.; McMullen, S. L.; Gillmor, D.; Apseloff, G.; Atiee, G.; Corsino, L.; Morrow, L.; Feldman, P. L. PLoS One 2014, 9, 1.
[6] White, M. F.; Kahn, C. R. J. Biol. Chem. 1996, 269, 1.
[7] Kenner, K. A.; Anyanwu, E.; Olefsky, J. M. J. Biol. Chem. 1996, 271, 19810.
[8] Walchli, S.; Curchod, M. L.; Gobert, R. P. J. Biol. Chem. 2000, 275, 9792.
[9] Sun, L.-P.; Ma, W.-P.; Gao, L.-X.; Yang, L.-L.; Quan, Y.-C.; Li, J.; Piao, H.-R. J. Enzyme Inhib. Med. Chem. 2013, 28, 1199.
[10] Sun, L.-P.; Jiang, Z.; Gao, L.-X.; Li, Y.-Z.; Sun, L.-Y.; Li, J.; Piao, H.-R. Chin. J. Org. Chem. 2012, 32, 2108 (in Chinese). (孙良鹏, 姜哲, 高立信, 李英哲, 孙立云, 李佳, 朴虎日, 有机化学, 2012, 32, 2108.)
[11] Chen, Y.-T.; Tang, C.-L.; Ma, W.-P.; Gao, L.-X.; Wei, Y.; Zhang, W.; Li, J.-Y.; Li, J.; Nan, F.-J. Eur. J. Med. Chem. 2013, 69, 399.
[12] Zhou, X.-W.; Tang, Y.-T.; Sang, F.; Zhang, X.-L.; Li, L.-X.; Zhou, H.-G.; Liu, W.; Dai, Y.-J. Chin. J. Org. Chem. 2015, 35, 1363 (in Chinese). (周晓伟, 唐延婷, 桑锋, 张秀利, 李立新, 周红刚, 刘伟, 戴玉洁, 有机化学, 2015, 35, 1363.)
[13] Mitchell F. R. Pharmacol. Biochem. Behav. 2010, 97, 138.
[14] Qin, Z.-H.; Pandey, N. R.; Zhou, X. Biochem. Biophys. Res. Commun. 2015, 458, 21.
[15] Zhang, W.; Hong, D.; Zhou, Y.; Zhang, Y.; Shen, Q.; Li, J.-Y.; Hu, L.-H.; Li, J. Biochim. Biophys. Acta, Gen. Subj. 2006, 1760, 1505.
[16] Qiu, W.-W.; Shen, Q.; Yang, F.; Wang, B.; Zou, H.; Li, J.-Y.; Li, J.; Tang, J. Bioorg. Med. Chem. Lett. 2009, 19, 6618.
[17] Li, H.; Zou, H.; Gao, L.-X.; Liu, T.; Yang, F., Li, J.-Y.; Li, J.; Qiu, W.-W.; Tang, J. Heterocycles 2012, 85, 1117.
[18] He, H.-B.; Gao, L.-X.; Deng, Q.-F.; Ma, W.-P.; Tang, C.-L.; Qiu, W.-W.; Tang, J.; Li, J.-Y.; Li, J.; Yang, F. Bioorg. Med. Chem. Lett. 2012, 22, 7237.
[19] Miro, P.; Vaya, I.; Sastre, G.; Jimenez, M. C.; Marin, M. L.; Miranda, M. A. Chem. Commun. 2016, 52, 713.
[20] Iida, T.; Kakiyama, G.; Hibiya, Y.; Miyata, S.; Inoue, T.; Ohno, K.; Goto, T.; Mano, N.; Goto, J.; Nambara, T.; Hofmann, A. F. Steroids 2006, 71, 18.
[21] Xia, P.; Yang, Z.-Y.; Xia, Y.; Zheng, Y.-Q.; Cosentino, L. M.; Lee, K.-H. Bioorg. Med. Chem. 1999, 7, 1907.
[22] Renata, H.; Zhou, Q.; Dünstl, G.; Felding, J.; Merchant, R. R.; Yeh, C.-H.; Baran, P. S. J. Am. Chem. Soc. 2015, 137, 1330.
[23] Upasani, R. B.; Harrison, B. L.; Askew, B. C. Jr.; Dodart, J.-C.; Salituro, F. G.; Robichaud, A. J. WO 2013036835, 2013[Chem. Abstr. 2013, 158, 447158].
[24] Foot, J. S.; Phillis, A. T.; Sharp, P. P.; Willis, A. C.; Banwell, M. G. Tetrahedron Lett. 2006, 47, 6817.
[25] Puius, Y. A.; Zhao, Y.; Sullivan, M.; Lawrence, D. S.; Almo, S. C.; Zhang, Z.-Y. Proc. Natl. Acad. Sci. U. S. A. 1997, 94, 13420.
[26] Shi, L.; Yu, H. P.; Zhou, Y. Y.; Du, J. Q.; Shen, Q.; Li, J. Y.; Li, J. Acta Pharmacol. Sin. 2008, 29, 278.
/
| 〈 |
|
〉 |