

2-(2-苯并呋喃基)-6-氯-3-喹啉甲酸衍生物的简便合成
收稿日期: 2016-04-28
修回日期: 2016-07-12
网络出版日期: 2016-08-10
基金资助
国家自然科学基金(Nos. 21476028,21402011)资助项目.
A Facile Synthesis of 2-(Benzofuran-2-yl)-6-chloroquinoline-3-carboxylic Acid Derivatives
Received date: 2016-04-28
Revised date: 2016-07-12
Online published: 2016-08-10
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21476028, 21402011).
以6-氯-2-氯甲基-3-喹啉甲酸乙酯(1)为底物,在无水碳酸钾作为缚酸剂、乙腈作为溶剂、聚乙二醇-400作为相转移催化剂的条件下分别与水杨醛(2a~2i)、邻羟基苯乙酮(2j~2o)及2-羟基-1-萘醛(2p)经“三步一锅法”在超声辅助下回流反应,成功的合成了2-(2-苯(萘)并呋喃基)-6-氯-3-喹啉甲酸衍生物(3a~3p).所合成化合物的结构均通过红外光谱、核磁共振氢谱、核磁共振碳谱和高分辨质谱得以证实.
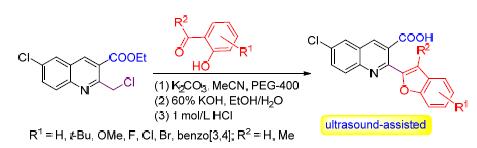
关键词: 6-氯-2-氯甲基-3-喹啉甲酸乙酯; 苯(萘)并呋喃; 聚乙二醇-400; 超声波辅助; 三步一锅法
高文涛 , 符鑫博 , 李阳 , 王东方 , 赵雅楠 . 2-(2-苯并呋喃基)-6-氯-3-喹啉甲酸衍生物的简便合成[J]. 有机化学, 2016 , 36(12) : 2965 -2972 . DOI: 10.6023/cjoc201604058
A facile synthesis of 2-(benzo(naphtho)furan-2-yl)-6-chloroquinoline-3-carboxylic acid derivatives (3a~p) has been described, involving ultrasound-assisted three-step one-pot reaction of ethyl 6-chloro-2-(chloromethyl)quinoline-3-carboxylate (1) with corresponding salicylaldehydes (2a~2i), 1-(2-hydroxyphenyl)ethanones (2j~2o) or 2-hydroxy-1-naphthaldehyde (2p) in the presence of K2CO3 as acid-binding agent and PEG-400 as phase transfer catalyst in refluxing MeCN. The synthesized compounds were new, and their structures had been determined by IR, 1H NMR, 13C NMR and HRMS.

[1] Michael, J. P. Nat. Prod. Rep. 1997, 14, 605.
[2] Wall, M. E.; Wani, M. C.; Cook, C. E.; Palmer, K. H.; McPhail, A. T.; Sim, G. A. J. Am. Chem. Soc. 1966, 88, 3888.
[3] Jain, P. P.; Degani, M. S.; Raju, A.; Anantram, A.; Seervi, M.; Sathaye, S.; Ray, M.; Rajan, M. G. R. Bioorg. Med. Chem. Lett. 2016, 26, 645.
[4] Patel, S. R.; Gangwal, R.; Sangamwar, A. T.; Jain, R. Eur. J. Med. Chem. 2015, 93, 511.
[5] Nayak, N.; Ramprasad, J.; Dalimba, U. J. Fluorine Chem. 2016, 183, 59.
[6] Muscia, G. C.; Buldain, G. Y.; Asis, S. E. Eur. J. Med. Chem. 2014, 73, 243.
[7] Joshi, M. C.; Wicht, K. J.; Taylor, D.; Hunter, R.; Smith, P. J.; Egan, T. J. Eur. J. Med. Chem. 2013, 69, 338.
[8] Kumar, A.; Srivastava, K.; Kumar, S. R.; Puri, S. K.; Chauhan, P. M. S. Bioorg. Med. Chem. Lett. 2010, 20, 7059.
[9] Kaur, K.; Jain, M.; Reddy, R. P.; Jain, R. Eur. J. Med. Chem. 2010, 45, 3245.
[10] Shobeiri, N.; Rashedi, M.; Mosaffa, F.; Zarghi, A.; Ghandadi, M.; Ghasemi, A.; Ghodsi, R. Eur. J. Med. Chem. 2016, 114, 14.
[11] Afzal, O.; Kumar, S.; Haider, M. R.; Ali, M. R.; Kumar, R.; Jaggi, M.; Bawa, S. Eur. J. Med. Chem. 2015, 97, 871.
[12] Chen, M.; Chen, H.; Ma, J. W.; Liu, X. Y.; Zhang, S. Y. Bioorg. Med. Chem. Lett. 2014, 24, 2867.
[13] Makawana, J. A.; Sangani, C. B.; Lin, L.; Zhu, H. L. Bioorg. Med. Chem. Lett. 2014, 24, 1734.
[14] Arafa, R. K.; Hegazy, G. H.; Piazza, G. A.; Abadi, A. H. Eur. J. Med. Chem. 2013, 63, 826.
[15] Nayak, N.; Ramprasad, J.; Dalimba, U. J. Fluorine Chem. 2016, 183, 59.
[16] Dolan, N.; Gavin, D. P.; Eshwika, A.; Kavanagh, K.; McGinley, J.; Stephens, J. C. Bioorg. Med. Chem. Lett. 2016, 26, 630.
[17] Abdullah, M. I.; Mahmood, A.; Madni, M.; Masood, S.; Kashif, M. Bioorg. Chem. 2014, 54, 31.
[18] Tabatabaeian, K.; Shojaei, A. F.; Shirini, F.; Hejazi, S. Z.; Rassa, M. Chin. Chem. Lett. 2014, 25, 308.
[19] Carlsson, B.; Singh, B. N.; Temciuc, M.; Nilsson, S.; Li, Y. L.; Mellin, C.; Malm, J. J. Med. Chem. 2002, 45, 623.
[20] Shirota, O.; Pathak, V.; Sekita, S.; Satake, M.; Nagashima, Y.; Hirayama, Y.; Hakamata, Y; Hayashi, T. J. Nat. Prod. 2003, 66, 1128.
[21] Wine-show, S.; Ian-Lih, T.; Teng, T. I.; Chen, I. S. Phyto-chemistry 1994, 36, 213.
[22] Mann, I. S.; Widdowson, D. A.; Clough, J. M. Tetrahedron 1991, 47, 7981.
[23] Gordaliza, M.; Castro, M. A.; Corral, J. M.; Lopez-Vazquez, M.; Feliciano, A. S.; Faircloth, G. T. Bioorg. Med. Chem. Lett. 1997, 7, 2781.
[24] Fuganti, C.; Serra, S. Tetrahedron Lett. 1998, 39, 5609.
[25] Abdel-Wahab, B. F.; Abdel-Aziz, H. A.; Ahmed, E. M. Eur. J. Med. Chem. 2009, 44, 2632.
[26] Kawasaki, K.; Masubuchi, M.; Morikami, K.; Sogabe, S.; Aoyama, T.; Ebiike, H.; Niizuma, S.; Hayase, M.; Fujii, T.; Sakata, K.; Shindoh, H.; Shiratori, Y.; Aoki, Y.; Ohtsuka, T.; Shimma, N. Bioorg. Med. Chem. Lett. 2003, 13, 87.
[27] Foster, R. T.; Robertson, A.; Bushra, A. J. Chem. Soc. 1948, 2254.
[28] Yue, D.; Yao, T.; Larock, R. C. J. Org. Chem. 2005, 70, 10292.
[29] Dawei, Y.; Larock, R. C. J. Org. Chem. 2002, 67, 1905.
[30] Larock, R. C.; Harrison, L. W. J. Am. Chem. Soc. 2002, 106, 188.
[31] Ghorbani-Vagher, R.; Akbari-Dadamahaleh, S. Tetrahedron Lett. 2009, 50, 1055.
[32] Wang, G. W.; Jia, C. S.; Dong, Y. W. Tetrahedron Lett. 2006, 47, 1059.
[33] Wang, J.; Jiang, F. C. Chin. J. Org. Chem. 2002, 22, 212(in Chinese).(王静, 姜凤超, 有机化学, 2002, 22, 212.)
[34] Zhang, X. B.; Wang, J. L.; Zhang, Y. L.; Ye, H. Chem. Reag. 2006, 28, 593(in Chinese).(张新波, 王家龙, 张雅娟, 叶红, 化学试剂, 2006, 28, 593.)
[35] Gao, W. T.; Liu, J.; Jiang, Y.; Li, Y. Beilstein J. Org. Chem. 2011, 7, 210.
[36] Gao, W. T.; Cheng, X. P.; Li, Y. Chin. J. Org. Chem. 2010, 30, 456(in Chinese).(高文涛, 程秀萍, 李阳, 有机化学, 2010, 30, 456.)
[37] Atar, A. B.; Dindulkar, S. D.; Jeong, Y. T. Monatsh. Chem. 2013, 144, 695.
[38] Gao, W. T.; Fu, X. B.; Li, K.; Wang, D. F.; Zhao, Y. N.; Li, Y. Chem. Res. Appl. 2015, 27, 1463(in Chinese).(高文涛, 符鑫博, 李凯, 王东方, 赵雅楠, 李阳, 化学研究与应用, 2015, 27, 1463.)
[39] Gao, W. T.; Tao, X. Y.; Lin, G. H.; Li, Y. Chin. J. Org. Chem. 2012, 32, 2171(in Chinese).(高文涛, 陶希月, 林贵海, 李阳, 有机化学, 2012, 32, 2171.)
[40] Gao, W. T.; Zhang, C. H.; Li, Y.; Jiang, Y. Chin. J. Org. Chem. 2009, 29, 1423(in Chinese).(高文涛, 张朝花, 李阳, 姜云, 有机化学, 2009, 29, 1423.)
[41] Stipanovic, R. D.; Bell, A. A.; Howell, C. R. Phytochemistry 1975, 14, 1809.
[42] Tatum, J. H.; Baker, R. A.; Berry, R. E. Phytochemistry 1987, 26, 2499.
[43] Hagiwara, H.; Sato, K.; Suzuki, T.; Ando, M. Heterocycles 1999, 51, 497.
/
| 〈 |
|
〉 |