

有效霉烯胺的化学合成进展
收稿日期: 2016-05-11
修回日期: 2016-07-08
网络出版日期: 2016-08-10
基金资助
国家自然科学基金(Nos. 21376213,21476194)、教育部高等学校博士学科点专项科研基金(No. 20120101110062)、浙江省博士后科研项目择优(No. BSH1502096)资助项目.
Review of Total Synthesis of Valienamine
Received date: 2016-05-11
Revised date: 2016-07-08
Online published: 2016-08-10
Supported by
Project supported by the National Science Foundation of China (Nos. 21376213, 21476194), the Doctoral Program of Higher Education of China (No. 20120101110062), and the Postdoctoral Advanced Programs of Zhejiang Province (No. BSH1502096).
计立 , 吴国锋 , 叶伟东 , 陈新志 . 有效霉烯胺的化学合成进展[J]. 有机化学, 2016 , 36(12) : 2771 -2785 . DOI: 10.6023/cjoc201605015
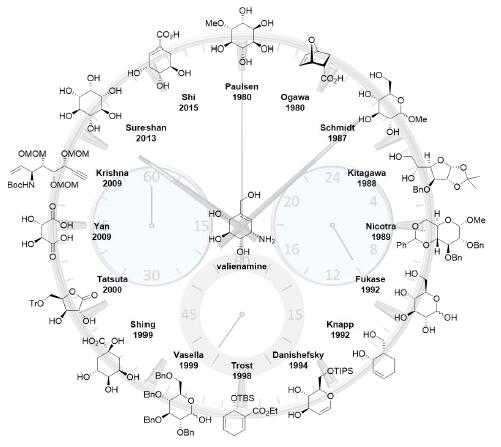
Valienamine is an α-glucosidase inhibitor belonging to C7N aminocyclitols that was isolated from the microbial degradation of validoxylamine A with Pseudomonas denitrificans. Moreover, valienamine is also an essential core unit in many kinds of pseudo-oligosaccharidic α-D-glucosidase inhibitors such as validamycins and acarbose. Due to the unique structural feature and interesting biological property, since the first isolation in 1972, a great deal of effort has been devoted to the development of various approaches for the efficient synthesis of valienamine. This review summarizes the 23 synthetic approaches towards valienamine reported in the last thirty years.
[1] Delgado, A. Eur. J. Org. Chem. 2008, 3893.
[2] Diaz, L.; Delgado, A. Curr. Med. Chem. 2010, 17, 2393.
[3] Arjona, O.; Gómez, A. M.; López, J. C.; Plumet, J. Chem. Rev. 2007, 107, 1919.
[4] Mahmud, T. Nat. Prod. Rep. 2003, 20, 137.
[5] Kameda, Y.; Horii, S. J. Chem. Soc., Chem. Commun. 1972, 1972, 746.
[6] Kamiya, K.; Wada, Y.; Horii, S.; Nishikawa, M. J. Antibiot. 1971, 24, 317.
[7] Fukase, H.; Horii, S. J. Org. Chem. 1992, 57, 3651.
[8] Chen, X. L.; Zheng, Y. G.; Shen, Y. C. Curr. Med. Chem. 2006, 13, 109.
[9] Chen, X. L.; Fan, Y. X.; Zheng, Y. G.; Shen, Y. C. Chem. Rev. 2003, 103, 1955.
[10] Fukuhara, K.; Murai, H.; Murao, S. Agric. Biol. Chem. 1982, 46, 2021.
[11] Truscheit, E.; Frommer, W.; Junge, B.; Müller, L.; Schmidt, D. D.; Wingender, W. Angew. Chem., Int. Ed. Engl. 1981, 20, 744.
[12] Kameda, Y.; Asano, N.; Yoshikawa, M.; Matsui, K. J. Antibiot. 1980, 33, 1575.
[13] Kameda, Y.; Asano, N.; Yoshikawa, M.; Takeuchi, M.; Yama-guchi, T.; Matsui, K.; Horii, S.; Fukase, H. J. Antibiot. 1984, 37, 1301.
[14] Horii, S.; Fukase, H.; Kameda, Y. Carbohydr. Res. 1985, 140, 185.
[15] Ji, L.; Zhang, D.-F.; Zhao, Q.; Hu, S.-M.; Qian, C.; Chen, X.-Z. Tetrahedron 2013, 69, 7031.
[16] Tanaka, K. S.; Winters, G. C.; Batchelor, R. J.; Einstein, F. W.; Bennet, A. J. J. Am. Chem. Soc. 2001, 123, 998.
[17] Wang, Y.; Bennet, A. J. Org. Biomol. Chem. 2007, 5, 1731.
[18] Horii, S.; Iwasa, T.; Kameda, Y. J. Antibiot. 1971, 24, 57.
[19] Berecibar, A.; Grandjean, C.; Siriwardena, A. Chem. Rev. 1999, 99, 779.
[20] Ji, L.; Zhou, G.-Q.; Qian, C.; Chen, X.-Z. Eur. J. Org. Chem. 2014, 3622.
[21] Paulsen, H.; Heiker, F. R. Angew. Chem., Int. Ed. Engl. 1980, 19, 904.
[22] Knapp, S.; Naughton, A. B. J.; Dhar, T. G. M. Tetrahedron Lett. 1992, 33, 1025.
[23] Shing, T. K.; Li, T. Y.; Kok, S. H.-L. J. Org. Chem. 1999, 64, 1941.
[24] Kok, S. H.-L.; Lee, C. C.; Shing, T. K. M. J. Org. Chem. 2001, 66, 7184.
[25] Mondal, S.; Prathap, A.; Sureshan, K. M. J. Org. Chem. 2013, 78, 7690.
[26] Nie, L.-D.; Shi, X.-X.; Ko, K. H.; Lu, W.-D. J. Org. Chem. 2009, 74, 3970.
[27] Quan, N.; Nie, L.; Shi, X.; Zhu, R.; Lü, X. Chin. J. Chem. 2012, 30, 2759.
[28] Quan, N.; Nie, L. D.; Zhu, R. H.; Shi, X. X.; Ding, W.; Lu, X. Eur. J. Org. Chem. 2013, 6389.
[29] Ding, W.; Yu, J. P.; Shi, X. X.; Nie, L. D.; Quan, N.; Li, F. L. Tetrahedron:Asymmetry 2015, 26, 1037.
[30] Ogawa, S.; Toyokuni, T.; Suami, T. Chem. Lett. 1980, 9, 713.
[31] Toyokuni, T.; Ogawa, S.; Suami, T. Bull. Chem. Soc. Jpn. 1983, 56, 1161.
[32] Ogawa, S.; Shibata, Y.; Nose, T.; Suami, T. Bull. Chem. Soc. Jpn. 1985, 58, 3387.
[33] Trost, B. M.; Chupak, L. S.; Lubbers, T. J. Am. Chem. Soc. 1998, 120, 1732.
[34] Trost, B. M. Chem. Pharm. Bull. 2002, 50, 1.
[35] Schmidt, R. R.; Köhn, A. Angew. Chem., Int. Ed. Engl. 1987, 26, 482.
[36] Nicotra, F.; Panza, L.; Ronchetti, F.; Russo, G. Gazz. Chim. Ital. 1989, 119, 577.
[37] Park, T. K.; Danishefsky, S. J. Tetrahedron Lett. 1994, 35, 2667.
[38] Chang, Y.-K.; Lo, H.-J.; Yan, T.-H. Org. Lett. 2009, 11, 4278.
[39] Yoshikawa, M.; Cha, B. C.; Nakae, T.; Kitagawa, I. Chem. Pharm. Bull. 1988, 36, 3714.
[40] Yoshikawa, M.; Cha, B. C.; Okaichi, Y.; Takinami, Y.; Yokokawa, Y.; Kitagawa, I. Chem. Pharm. Bull. 1988, 36, 4236.
[41] Shing, T. K.; Chen, Y.; Ng, W. Synlett 2011, 1318.
[42] Shing, T. K. M.; Chen, Y.; Ng, W.-L. Tetrahedron 2011, 67, 6001.
[43] Tatsuta, K.; Mukai, H.; Takahashi, M. J. Antibiot. 2000, 53, 430.
[44] Zhou, B.; Luo, Z.; Lin, S.; Li, Y. C. Synlett 2012, 913.
[45] Plumet, J.; Gomez, A. M.; Lopez, J. C. Mini-Rev. Org. Chem. 2007, 4, 201.
[46] Kapferer, P.; Sarabia, F.; Vasella, A. Helv. Chim. Acta 1999, 82, 645.
[47] Chang, Y.-K.; Lee, B.-Y.; Kim, D. J.; Lee, G. S.; Jeon, H. B.; Kim, K. S. J. Org. Chem. 2005, 70, 3299.
[48] Cumpstey, I. Tetrahedron Lett. 2005, 46, 6257.
[49] Cumpstey, I.; Gehrke, S.; Erfan, S.; Cribiu, R. Carbohydr. Res. 2008, 343, 1675.
[50] Li, Q. R.; Kim, S. I.; Park, S. J.; Yang, H. R.; Baek, A. R.; Kim, I. S.; Jung, Y. H. Tetrahedron 2013, 69, 10384.
[51] Lo, H.-J.; Chen, C.-Y.; Zheng, W.-L.; Yeh, S.-M.; Yan, T.-H. Eur. J. Org. Chem. 2012, 2780.
[52] Krishna, P. R.; Reddy, P. S. Synlett 2009, 209.
/
| 〈 |
|
〉 |