

2-嘧啶氧基-N-芳基苄胺类衍生物的酸催化Smiles重排反应条件的快速筛选和产物的高效制备
收稿日期: 2016-04-10
修回日期: 2016-06-02
网络出版日期: 2016-08-10
基金资助
公益性行业(农业)科研专项(No. 201403030)和国家自然科学基金(No. 31471807)资助项目.
A Fast and Efficient Approach for Screening and Synthesis of the Products of 2-Pyrimidinyloxy-N-arylbenzylamine Derivatives via Acid-Catalyzed Smiles Rearrangement
Received date: 2016-04-10
Revised date: 2016-06-02
Online published: 2016-08-10
Supported by
Project supported by the Agro-scientific Research in the Public Interest (No. 201403030) and the National Natural Science Foundation of China (No. 31471807).
在室温、甲醇水溶剂条件下,以盐酸为酸催化剂,以2-嘧啶氧基-N-芳基苄胺衍生物为反应原料,基于高效液相色谱(HPLC)监测,能快速、高效、便捷地实现Smiles重排反应.本方法的优点在于反应体系溶剂可重复利用,在无需分离的条件下即可取得良好的产率和纯度.从催化剂和溶剂的廉价以及反应的高效简便和绿色温和角度考虑,该合成方法具有较好的实用性和绿色经济性.
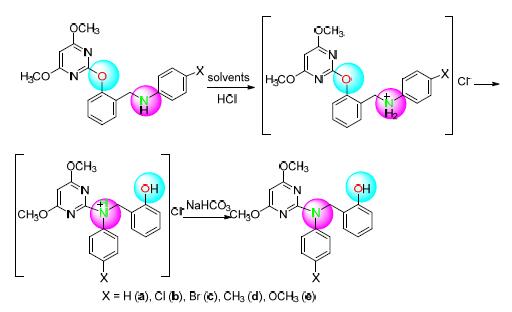
关键词: 2-嘧啶氧基-N-芳基苄胺类化合物; Smiles重排反应; 酸催化反应; 取代基效应; 高效液相色谱
叶美君 , 张培志 , 吴军 . 2-嘧啶氧基-N-芳基苄胺类衍生物的酸催化Smiles重排反应条件的快速筛选和产物的高效制备[J]. 有机化学, 2016 , 36(12) : 2997 -3002 . DOI: 10.6023/cjoc201604020
Based on high performance liquid chromatography (HPLC), a fast, efficient and simple route for Smiles rearrangement reaction has been developed in the presence of 2-pyrimidinyloxy-N-arylbenzylamine derivatives as reactants with HCl as acid catalyst in mixed CH3OH/H2O medium at room temperature. The advantage of the proposed method is that the products with excellent yields and high purity can be obtained employing reused solvent without further separating process. Considering the cheapness of catalyst and solvent, as well as high efficient and environment-friendly reaction condition, the developed method has the practicability and environmentally friendliness.

[1] Wu, J.; Cheng, J.; Lu, L. J. Agric. Food Chem. 2006, 54, 5954.
[2] Wu, J.; Zhang, P. Z.; Lu, L.; Yu, Q. S.; Hu, X. R.; Gu, J. M. Chin. J. Struct. Chem. 2003, 22, 613.
[3] Fan, Q.-J.; Peng, W.-L.; Shen, D.-L. J. Agrochemicals 2005, 44, 257(in Chinese).(范钱君, 彭伟立, 沈德隆, 农药, 2005, 44, 257.)
[4] Zhang, Q.; Hang, J.-H.; Yang, Z.-M.; Zhu, Q.-S.; Ye, Q.-F.; Lu, L; Xu, B.-J.; Chen, Z.-F. Acta Agric. Nucl. Sin. 2008, 22, 84(in Chinese).(张泉, 黄建中, 杨征敏, 朱其松, 叶庆富, 吕龙, 徐步进, 陈子元, 核农学报, 2008, 22, 84.)
[5] Zhang, Q. M.S. Thesis, Zhejiang University, Hangzhou, 2007(in Chinese).(张泉, 硕士论文, 浙江大学, 杭州, 2007.)
[6] Wang, H. Y.; Liao, Y. X.; Guo, Y. L.; Tang, Q. H.; Lu, L. Synlett 2005, 1239.
[7] Kitching, M. O.; Hurst, T. E.; Sniekus, V. Angew. Chem., Int. Ed. 2012, 51, 2925.
[8] Yu, J. Z.; Wang, Y. T.; Zhang, P. Z.; Wu, J. Synlett 2013, 24, 1448.
[9] Yu, J. Z.; Zhang, P. Z.; Wu, J.; Shang, Z. C. Tetrahedron Lett. 2013, 24, 3167.
[10] Liu, S. H.; Hu, Y.; Qian, P. F.; Hu, Y. W.; Ao, G. Z.; Chen, S. H.; Zhang, S. L.; Zhang, Y. N. Tetrahedron Lett. 2015, 56, 2211
[11] Nechepurenko, I. V.; Komarova, N. I.; Shernyukov, A. V.; Vasiliev, V. G.; Salakhutdinov, N. F. Tetrahedron Lett. 2014, 55, 6125
[12] Xiao, Y. X.; Zhang, Z. C.; Chen, Y. B.; Shao, X. S.; Li, Z.; Xu, X. Y. Tetrahedron 2015, 71, 1863.
[13] Takahashi, T.; Maki, Y. Chem. Pharm. Bull. 1958, 6, 369.
[14] Rodig, O. R.; Schlatze, R. K.; Collier, R. E. J. Org. Chem. 1964, 29, 2652.
[15] Sunamoto, J.; Kondo, H.; Yanase, F.; Okamoto, H. B. Chem. Soc. Jpn. 1980, 53, 1361.
[16] Lindberg, P.; Nordberg, P.; Alminger, T.; Brandstrom, A.; Wallmark, B. J. Med. Chem. 1986, 29, 1327.
[17] Terauchi, H.; Tanitame, A.; Tada, K.; Nakamura, K.; Seto, Y.; Nishikawa, Y. J. Med. Chem. 1997, 40, 313.
[18] Kuhler, T. C.; Swanson, M.; Christenson, B.; Klintenberg, A. C.; Lamm, B.; Fagerhag, J.; Gatti, V.; Elebring, T. J. Med. Chem. 2002, 45, 4282.
[19] Potashman, M. H.; Duggan, M. E. J. Med. Chem. 2009, 52, 1231.
[20] Shin, J. M.; Cho, Y. M.; Sachs, G. J. Am. Chem. Soc. 2004, 126, 7800.
[21] Kürti, L.; Czakó, B. Strategic Applications of Named Reactions in Organic Synthesis, Elsevier Academic Press, New York, 1961, pp. 417~418.
[22] Plesniak, K.; Zarecki, A.; Wicha, J. Top. Curr. Chem. 2007, 275, 163.
[23] Selvakumar, N.; Srinivas, D.; Azhagan, A. M.; Selvakumar, N. Synthesis 2002, 2421.
[24] Cho, S. D.; Park, Y. D.; Kim, J. J.; Lee, S. G.; Ma, C.; Song, S. Y.; Joo, W. H.; Falck, J. R.; Shiro, M.; Shin, D. S.; Yoon, Y. J. J. Org. Chem. 2003, 68, 7918.
[25] Mosselhi, M. A. N.; Abdallah, M. A.; Farghaly, T. A.; Shawalli, A. S. Monatsh. Chem. 2004, 135, 211.
[26] Xiang, J.; Zheng, L.; Xie, H.; Hu, X.; Dang, Q.; Bai, X. Tetrahedron 2008, 64, 9101.
[27] Yu, J.-Z. Ph.D. Dissertation, Zhejiang University, Hangzhou, 2013(in Chinese).(俞建忠, 博士论文, 浙江大学, 杭州, 2013.)
[28] Zhang, P.-Z.; Ye, M.-J.; Hu, W.-L.; Wu, J. Acta Phys.-Chim. Sin. 2016, 32, 422(in Chinese).(张培志, 叶美君, 胡伟莲, 吴军, 物理化学学报, 2016, 32, 422.)
[29] Wu, H. F.; Zhang, P. Z.; Wu, J. J. Zhejiang Univ., Sci. Ed. 2010, 11, 94.
/
| 〈 |
|
〉 |