

手性Binap-Ru(II)催化剂用于不对称加氢反应的研究进展
收稿日期: 2016-06-14
修回日期: 2016-08-12
网络出版日期: 2016-09-01
基金资助
国家“十二五”科技支撑计划课题(No. 2012BAE06B08)和云南省科技创新强者计划(Nos. 2015AA006,2013B019)资助项目.
Research Advances of the Chiral Binap-Ru(II) Catalysts in Asymmetric Hydrogenation Reactions
Received date: 2016-06-14
Revised date: 2016-08-12
Online published: 2016-09-01
Supported by
Project supported by the 12th National Scientific Support Plan(No. 2012BAE06B08) and the Yunnan Province Scientific Creative Plan(Nos. 2015AA006, 2013B019).
高安丽 , 叶青松 , 余娟 , 刘伟平 . 手性Binap-Ru(II)催化剂用于不对称加氢反应的研究进展[J]. 有机化学, 2017 , 37(1) : 47 -78 . DOI: 10.6023/cjoc201606024
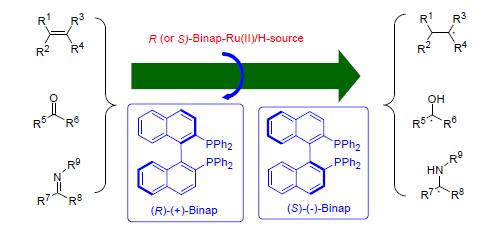
Chiral 2,2'-bis(diphenylphosphino)-1,1'-binapthyl(Binap) ligand consists of a pair of 2-diphenylphosphinonaphthyl groups connected at the 1 and 1'positions,being of the nature of the C2-axial chirality with optical activity of ±234°.The Binap can coordinate with many transitional metals to form stable chelation complexes,effecting the chirality transfer to the metal center for function in asymmetrically catalytic reactions.This contribution is focused on the Binap-Ru(Ⅱ) catalytic system that covers the various substrates,reaction conditions,enantioselectivity of the products,reaction mechanism,and others on the basis of the prepared stable Binap-Ru(Ⅱ) complexes as the clue in the text.This study will give a deep understanding of this system especially concerning the applied catalytic synthesis of the useful organic molecules.
[1] Miyashita, A.; Yasuda, A.; Takaya, H.; Toriumi, K.; Ito, T.; Souchi, T.; Noyori, R. J. Am. Chem. Soc. 1980, 102, 7932.
[2] Ikariya, T.; Ishii, Y.; Kawano, H.; Arai, T.; Saburi, M.; Yoshikawa, S.; Akutagawa, S. J. Chem. Soc., Chem. Commun. 1985, 922.
[3] Noyori, R.; Ohta, M.; Hsiao, Y.; Kitamura, M.; Ohta, T.; Takaya, H. J. Am. Chem. Soc. 1986, 108, 7 117.
[4] Kitamura, M.; Hsiao, Y.; Noyori, R. Takaya, H. Tetrahedron Lett. 1987, 28, 4829
[5] Kitamura, M.; Hsiao, Y.; Ohta, M.; Tsukamoto, M.; Ohta, T.; Takaya, H.; Noyori, R. J. Org. Chem. 1994, 59, 297.
[6] Ohta, T.; Takaya, H.; Kitamura, M.; Nagai, K.; Noyori, R. J. Org. Chem. 1987, 52, 3174.
[7] Takaya, H.; Ohta, T.; Sayo, N.; Kumobayashi, H.; Akutagawa, S.; Inoue, S.; Kasahara, I.; Noyori, R. J. Am. Chem. Soc. 1987, 109, 1596.
[8] Bernas, H.; Bernas, A.; Maki-Arvela, P.; Leino, R.; Murzin, Y. D. Catal. Sci. Technol. 2012, 2, 1901
[9] Kitamura, M.; Kasahara, I.; Manabe, K.; Noyori, R.; Takaya, H. J. Org. Chem. 1988, 53, 708..
[10] Ashby, M. T.; Khan, M. A. Organometallics 1991, 10, 2011.
[11] Ashby, M. T.; Halpern, J. J. Am. Chem. Soc. 1991, 113, 589.
[12] Manimaran, T.; Wu, T. C.; Klobucar, W. D.; Kolich, C. H.; Stahly, G. P. Organometallics 1993, 12, 1467.
[13] Chan, A. S.; Laneman, S. A.; Day, C. X. Inorg. Chim. Acta 1995, 228 159.
[14] Chen, C. C.; Huang, T. T.; Lin, C. W.; Cao, R.; Chan, A. S. C.; Wong, W. T. Inorg. Chim. Acta 1988, 270, 247.
[15] Ohta, T.; Miyake, T.; Seido, N.; Kumobayashi, H.; Takaya, H. J. Org. Chem. 1995, 60, 357.
[16] Ohta, T.; Ikegami, H.; Miyake, T.; Takaya, H. J. Organomet. Chem. 1995, 502, 169.
[17] Uemura, T.; Zhang, X. Y.; Matsumura, K.; Sayo, N.; Kumobayashi, H.; Ohta, T.; Nozaki, K.; Takaya, H. J. Org. Chem. 1996, 61, 5510.
[18] Marchetti, M.; Alberico, E.; Bertucci, C.; Botteghi, C.; Ponte, G. D. J. Mol. Catal. A:Chem. 1997, 125, 109.
[19] Shinohara, T.; Kondo, K.; Ogawa, H.; Mori, T.; Nozaki, K.; Hiyama, T. Chirality 2000, 12, 425.
[20] Kitamura, M.; Tsukamoto, M.; Bessho, Y.; Yoshimura, M.; Kobs, U.; Widhalm, M.; Noyori, R. J. Am. Chem. Soc. 2002, 124, 6649.
[21] Ciappa, A.; Matteoli, U.; Scrivanti, A. Tetrahedron:Asymmetry 2002, 13, 2193.
[22] Jessop, P. G.; Stanley, R. R.; Brown, R. A.; Eckert, C. A.; Liotta, C. L.; Ngo, T. T.; Pollet, P. Green Chem. 2003, 5, 123.
[23] Dong, X., Erkey, C. J. Mol. Catal. A:Chem. 2004, 211, 73.
[24] Yamano, T.; Yamashita, M.; Adachi, M.; Tanaka, M.; Matsumoto, K.; Kawada, M.; Uchikawa, O.; Fukatsu, K.; Ohkawa, S. Tetrahedron:Asymmetry 2006, 17, 184.
[25] Bisset, A. A.; Dishington, A.; Jones, T.; Clarkson, G. J.; Wills, M. Tetrahedron 2014, 70, 7207.
[26] Yoshimura, M.; Ishibashi, Y.; Miyata, K.; Bessho, Y.; Tsuka-moto, M.; Kitamura, M. Tetrahedron 2007, 63, 11399.
[27] Kitamura, M.; Tokunaga, M.; Noyori, R. J. Am. Chem. Soc. 1993, 115, 144.
[28] Doucet, H.; Gendre, P. L.; Bruneau, C.; Dixneuf, P. H.; Souvie, J. C. Tetrahedron:Asymmetry 1996, 7, 525
[29] Tranchier, J. P.; Ratovelomanana-Vidal, V.; Genet, J. P.; Tong, S. J.; Cohen, T. Tetrahedron Lett. 1997, 38, 2951.
[30] Magnus, N. A.; Astleford, B. A.; Laird, D. L. T.; Maloney, T. D.; McFarland, A. D.; Rizzo, J. R.; Ruble, J. C.; Stephenson, G. A.; Wepsiec, J. P. J. Org. Chem. 2013, 78, 5768.
[31] Charette, A. B.; Glroux, A. Tetrahedron Lett. 1996, 37, 6669.
[32] Tomas-Mendivil, E.; Menendez-Rodriguez, L.; Francos, J.; Crochet, P.; Cadierno, V. RSC Adv. 2014, 4, 63466.
[33] Mashima, K.; Matsumura, Y.; Kusano, K,; Kumobayashi, H.; Sayo, N.; Hori, Y.; Ishizaki, T.; Akutagawa, S.; Takaya, H. J. Chem. Soc., Chem. Commun. 1991, 609.
[34] Ohta, T.; Miyake, T.; Takaya, H. J. Chem. Soc., Chem. Commun. 1992, 1725.
[35] Gelman, F.; Avnir, D.; Schumann, H.; Blum, J. J. Mol. Catal. A:Chem. 1999, 146, 123.
[36] Bronze-Uhle, E. S.; Sairre, M. I.; Donate, P. M.; Frederico, D. J. Mol. Catal. A:Chem. 2006, 259, 103
[37] Mashima, K.; Kusano, K.; Sate, N.; Matsumura, Y.; Nozaki, K.; Kumobayashi, H.; Sayo, N.; Hori, Y.; Ishizaki, T.; Akutagawa, S.; Takaya, H. J. Org. Chem. 1994, 59, 3064.
[38] Schmidt, U.; Leitenberger, V.; Griesser, H.; Schmidt, J.; Meyer, R. Synthesis 1992, 1248.
[39] Rane, V. H.; Tas, D.; Parton, R. F.; Jacobs, P. A. Catal. Lett. 1996, 41, 111.
[40] Bianchini, C.; Barbaro, P.; Scapacci, G.; Zanobini, F. Organometallics 2000, 19, 2450.
[41] Wolfson, A,; Vankelecom, I. F. J.; Geresh, S.; Jacobs, P. A. J. Mol. Catal. A:Chem. 2004, 217, 21.
[42] Wolfson, A.; Vankelecom, I. F. J.; Jacobs, P. A. J. Organomet. Chem. 2005, 690, 3558.
[43] Ahn, S. H.; Park, Y. H.; Jacobs, P. A. Surf. Sci. Catal. 2006, 159, 349.
[44] Jahjah, M.; Alame, M.; Pellet-Rostaing, S.; Lemaire, M. Tetrahedron:Asymmetry 2007, 18, 2305.
[45] Floris, T.; Kluson, P.; Bartek, L.; Pelantova, H. Appl. Catal. A:Gen. 2009, 366, 160.
[46] Cerna, I.; Kluson, P.; Bendova, M.; Floris, T.; Pelantova, H.; Pekarek, T. Chem. Eng. Process. 2011, 50, 264.
[47] Theuerkauf, J.; Francio, G.; Leitner, W. Adv. Synth. Catal. 2013, 355, 209.
[48] Facchetti, S.; Jurcik, V.; Baldino, S.; Giboulot, S.; Nedden, H. G.; Zanotti-Gerosa, A.; Blackaby, A.; Bryan, R.; Boogaard, A.; McLaren, D. B.; Moya, E.; Reynolds, S.; Sandham, K. S.; Martinuzzi, P.; Baratta, W. Organometallics 2016, 35, 277.
[49] Kawano, H.; Ikariya, T.; Ishii, Y.; Saburi, M.; Yoshikawa, S.; Uchida, Y.; Kumobayashi, H. J. Chem. Soc., Perkin Trans. 1 1989, 1571.
[50] Muramatsu, H.; Kawano, H.; Ishii, Y.; Saburi, M.; Uchida, Y. J. Chem. Soc., Chem. Commun. I989, 769.
[51] Monteiro, A. L.; Zinn, F. K.; Souza, R. F.; Doupont, J. Tetrahe-dron:Asymmetry 1997, 8, 177.
[52] Yamamoto, T.; Ogura, M; Kanisawa, T. Tetrahedron 2002, 58, 9209.
[53] Kawano, H.; Ishii, Y.; Saburi, M.; Uchida, Y. J. Chem. Soc., Chem. Commun. 1988, 87.
[54] Furstner, A.; Thiel, O. R.; Blanda, G. Org. Lett. 2000, 2, 3731.
[55] Furstner, A.; Dierkes, T.; Thiel, O. R.; Blanda, G. Chem. Eur. J. 2001, 7, 5286.
[56] Zhao, Z. Q.; Zhou, Z. Y.; Peng, L. Z. Chin. J. Pharm. 2006, 37, 726(in Chinese).(赵志全, 周宗仪, 彭立增, 中国医药工业杂志, 2006, 37, 726.)
[57] Kramer, R.; Bruckner, R. Angew. Chem., Int. Ed. 2007, 46, 6537.
[58] Kramer, R.; Bruckner, R. Chem. Eur. J. 2007, 13, 9076.
[59] Nair, D.; Wong, H. T.; Han, S. J.; Vankelecom, I. F. J.; White, L. S.; Livingston, A. G.; Boam, A. T. Org. Process Res. Dev. 2009, 13, 863.
[60] Falkowski, J. M.; Sawano, T.; Zhang, T.; Tsun, G,; Chen, Y.; Lockard, J. V.; Lin, W. B. J. Am. Chem. Soc. 2014, 136, 5213.
[61] Kitamura, M.; Ohkuma, T.; Takaya, H.; Noyori, R. Tetrahedron Lett. 1988, 29, 1555.
[62] Genet, J. P.; Ratovelomanana-Vidal, V.; Andrade, M. C.; Pfister, X.; Guerreiro, P.; Lenoir, J. Y. Tetrahedron Lett. 1995, 36, 4801.
[63] Genet, J. P.; Andrade, M. C. C.; Ratovelomanana-Vidal, V. Tetrahedron Lett. 1995, 36, 2063.
[64] Shioiri, T.; Terao, Y.; Irako, N.; Aoyama, T. Tetrahedron 1998, 54, 15701.
[65] Makino, K.; Goto, T.; Hiroki, Y.; Hamada, Y. Angew. Chem., Int. Ed. 2004, 43, 882.
[66] Huang, H. L.; Liu, L. T.; Chen, S. F.; Ku, H. Tetrahedron:Asymmetry 1998, 9, 1637
[67] Kitamura, M.; Ohkuma, T.; Tokunaga, M.; Noyori, R. Tetrahedron Asymmetry 1990, 1, 1.
[68] Kitamura, M.; Okhuma, T.; Inoue, S.; Sayo, N.; Kumobayashi, H.; Akutagawa, S.; Ohta, T.; Takaya, H.; Noyori, R. J. Am. Chem. Soc. 1988, 110, 629.
[69] Kitamura, M.; Tokunaga, M.; Pham, T.; Lubell, W. D.; Noyori, R. Tetrahedron Lett. 1995, 36, 5769.
[70] Kitamura, M.; Yoshimura, M.; Kanda, N.; Noyori, R. Tetrahe-dron 1999, 55, 8769.
[71] Lamouille, T.; Saluzzo, C.; Halle, R.; Guyader, F. L.; Lemaire, M. Tetrahedron Lett. 2001, 42, 663.
[72] Berthod, M.; Saluzzo, C.; Mignani, G.; Lemaire, M. Tetrahedron:Asymmetry 2004, 15, 639.
[73] Ohkuma, T.; Ooka, H.; Hashiguchi, S.; Ikariya, T.; Noyori, R. J. Am. Chem. Soc. 1995, 117, 2675.
[74] Moreau, C.; Frost, C. G.; Murrer, B. Tetrahedron Lett. 1999, 40, 5617.
[75] Ohkuma, T.; Hattori, T.; Ooka, H.; Inoue, T.; Noyori, R. Org. Lett. 2004, 6, 2681.
[76] Liu, W. G.; Cui, X.; Cun, L. F.; Wu, J.; Zhu, J.; Deng, J. G.; Fan, Q. H. Synlett 2005, 1591.
[77] Jing, Q.; Zhang, X.; Sun, J.; Ding, K. Adv. Synth. Catal. 2005, 347, 1193.
[78] Arai, N.; Azuma, K.; Nii, N.; Ohkuma, T. Angew. Chem., Int. Ed. 2008, 47, 7457.
[79] Ooka, H.; Arai, N.; Azuma, K.; Kurono, N.; Ohkuma, T. J. Org. Chem. 2008, 73, 9084.
[80] Arai, N.; Suzuki, K.; Sugizaki, S.; Sorimachi, H.; Ohkuma, T. Angew. Chem., Int. Ed. 2008, 47, 1770.
[81] Sandoval, C. A.; Li, Y. H.; Ding, K. L.; Noyori, R. Chem. Asian J. 2008, 3, 1801.
[82] Chiwara, V. I.; Haraguchi, N.; Itsuno, S. J. Org. Chem. 2009, 74, 1391.
[83] Rivera, V. M.; Ruelas-Leyva, P. J.; Fuentes, G. A. Catal. Today 2013, 213, 109.
[84] Arai, N.; Sato, K.; Azuma, K.; Ohkuma, T. Angew. Chem., Int. Ed. 2013, 52, 7500.
[85] Boge, M.; Heck, J. Eur. J. Inorg. Chem. 2015, 2858.
[86] Ohkuma, T.; Koizumi, M.; Muniz, K.; Hilt, G.; Kabuto, C.; Noyori, R. J. Am. Chem. Soc. 2002, 124, 6508.
[87] Sandoval, C. A.; Shi, Q. X.; Liu, S. S.; Noyori, R. Chem. Asian J. 2009, 4, 1221.
[88] Sandoval, C. A.; Ohkuma, T.; Muniz, K.; Noyori, R. J. Am. Chem. Soc. 2003, 125, 13490.
[89] Hamilton, R. J.; Bergens, S. H. J. Am. Chem. Soc. 2008, 130, 11979.
[90] Sui-Seng, S.; Hadzovic, A.; Lough, A. J.; Morris, R. H. Dalton Trans. 2007, 2536.
[91] Takebayashi, S.; Bergens, S. H. Organometallics 2009, 28, 2349.
[92] Takebayashi, S.; John, J. M.; Bergens, S. H. J. Am. Chem. Soc. 2010, 132, 12832.
[93] Matsumura, K.; Arai, N.; Hori, K.; Saito, T.; Sayo, N.; Ohkuma, T. J. Am. Chem. Soc. 2011, 133, 10696.
[94] Faza, O. N.; Fernandez, I.; Lopez, C. S. Chem. Commun. 2013, 49, 4277.
[95] Guo, R. W.; Lough, A. J.; Morris, R. H.; Song, D. T. Organometallics 2004, 23, 5524.
[96] Abdur-Rashid, A.; Guo, R. W.; Lough, A. J.; Morris, R. H.; Song, D. T. Adv. Synth. Catal. 2005, 347, 571.
[97] Wiles, J. A.; Lee, C. E.; McDonald, R.; Bergens, S. H. Organometallics 1996, 15, 3782.
[98] Noyori, R.; Ikeda, T.; Ohkuma, T.; Widhalm, M.; Kitamura, M.; Takaya, H.; Akutagawa, S.; Sayo, N.; Saito, T.; Taketomi, T.; Kumobayashi, H. J. Am. Chem. Soc. 1989, 111, 9134.
[99] Miyashita, A.; Takaya, H.; Souchi, T.; Noyori, R. Tetrahedron 1984, 40, 1245.
[100] Wiles, J. A.; Bergens, S. H. Organometallics 1998, 17, 2228.
[101] Wiles, J. A.; Bergens, S. H.; Young, V. G. Can. J. Chem. 2001, 79, 1019.
[102] Wiles, J. A.; Daley, C. J. A.; Hamilton, R. J.; Leong, C. G.; Ber-gens, S. H. Organometallics 2004, 23, 4564.
[103] Daley, C. J. A.; Wiles, J. A.; Bergens, S. H. Can. J. Chem. 1998, 76, 1447.
[104] Daley, C. J. A.; Bergens, S. H. J. Am. Chem. Soc. 2002, 124, 3680.
[105] Daley, C. J. A.; Wiles, J. A.; Bergens, S. H. Inorg. Chim. Acta 2006, 359, 2760
[106] Kurono, N.; Uemura, M.; Ohkuma, T. Eur. J. Org. Chem. 2010, 1455.
[107] Kurono, N.; Nii, N.; Sakaguchi, Y.; Uemura, M.; Ohkuma, T. Angew. Chem., Int. Ed. 2011, 50, 5541.
[108] Uemura, M.; Kurono, N.; Sakai, Y.; Ohkuma, T. Adv. Synth. Catal. 2012, 354, 2023.
[109] Zhao, X. X.; Ivanova, N.; Hadzovic, A.; Iuliis, M. Z. D.; Lough, A. J.; Morris, R. H. Organometallics 2008, 27, 503.
[110] Ohta, T.; Tonomura, Y.; Nozaki, K.; Takaya, H.; Mashima, K. Organometallics 1996, 15, 1521.
[111] Matteoli, U.; Beghetto, V.; Scrivanti, A. J. Mol. Catal. A:Chem. 1996, 109, 45.
[112] Nispen, S. F. G. M.; Buijtenen, J.; Vekemans, J. A. J. M.; Meuldijkb, J.; Hulshof, L. A. Tetrahedron:Asymmetry 2006, 17, 2299.
[113] Taber, D. F.; Deker, P. B.; Silverberg, L. J. J. Org. Chem. 1992, 57, 5990.
[114] Xie, B. H.; Lu, S. J.; Gao, L. Y.; Fu, H. X. J. Mol. Catal.(China) 1997, 11, 433(in Chinese).(谢宝汉, 吕士杰, 高兰云, 傅宏祥, 分子催化, 1997, 11, 433.)
[115] Guerreiro, P.; Andrane, M. C. C.; Henry, J. C.; Tranchier, J. P.; Phansavath, P.; Ratovelomanana-Vidal, V.; Genet, J. P.; Homri, T.; Touati, A. R.; Hassine, B. B. Org. Organomet. Synth. 1999, 175.
[116] Birdsall, D. J.; Hope, E. G.; Stuart, A. M.; Chen, W. P.; Hu, Y. L.; Xiao, J. L. Tetrahedron Lett. 2001, 42, 8551.
[117] Berthod, M.; Mignani, G.; Lemaire, M. Tetrahedron:Asymmetry 2004, 15, 1121.
[118] Geldbach, T. J.; Pregosin, P, S. Helv. Chim. Acta 2002, 85, 3937.
[119] Thakur, V. V.; Nikalje, M. D.; Sudalai, A. Tetrahedron:Asym-metry 2003, 14, 581.
[120] Hu, Y. L.; Birdsall, D. J.; Stuart, A. M.; Hope, E. G.; Xiao, J. L. J. Mol. Catal. A:Chem. 2004, 219, 57.
[121] Starodubtseva, E. V.; Turova, O. V.; Vinogradov, M. G.; Gorshkova, L. S.; Ferapontov, V. A.; Struchkova, M. I. Tetrahedron 2008, 64, 11713.
[122] Kockritz, A.; Bischoff, S.; Kant, M.; Siefken, R. J. Mol. Catal. A:Chem. 2001, 174, 119.
[123] Seki, T.; McEleney, K.; Crudden, C. M. Chem. Commun. 2012, 48, 6369.
[124] Xu, J. Y.; Ou, Z. M.; Yang, G. S. Zhejiang Chem. Ind. 2010, 41, 20(in Chinese).(徐姜炎, 欧志敏, 杨根生, 浙江化工, 2010, 41, 20.)
[125] Horn, J.; Bannwarth, W. Eur. J. Org. Chem. 2007, 2058.
[126] Keshavarz, E.; Tabatabaeian, K.; Mamaghani, M.; Mahmoodi, N. O. Curr. Chem. Lett. 2012, 1, 91.
[127] Fan, Q. H.; Chen, Y. M.; Chen, X. M.; Jiang, D. Z.; Xi, F.; Chan, A. S. C. Chem. Commun. 2000, 789.
[128] Deng, G. J.; Fan, Q. H.; Chen, X. M.; Liu, D, S.; Chan, A. S. C. Chem. Commun. 2002, 1570.
[129] Deng, G. J.; Yi, B.; Huang, Y. Y.; Tang, W. J.; He, Y. M.; Fan, Q. H. Adv. Synth. Catal. 2004, 346, 1440.
[130] Huang, Y. Y.; He, Y. M.; Zhou, H. F.; Wu, L.; Li, B. L.; Fan, Q. H. J. Org. Chem. 2006, 71, 2874.
[131] Ma, B. D.; Deng, G. J.; Liu, J.; He, Y. M.; Fan, Q. H. Acta Chim. Sinica 2013, 71, 528(in Chinese).(马保德, 邓国军, 刘继, 何艳梅, 范青华, 化学学报, 2013, 71, 528.)
[132] Huang, Y. Y.; Yang, X.; Feng, Y.; Verpoort, F.; Fan, Q. F. J. Mol. Catal. A:Chem. 2014, 393, 150.
[133] Liu, J.; Ma, B. D.; Feng, Y.; He, Y. M.; Fan, Q. H. Inorg. Chim. Acta 2014, 409, 106.
[134] Schinkel, M.; Wang, L. H.; Bielefeld, K.; Ackermann, L. Org. Lett. 2014, 16, 1876.
[135] Wang, X.; Lu, S. M.; Li, J.; Liu, Y.; Li, C. Catal. Sci. Technol. 2015, 5, 2585.
[136] Zhao, B. G.; Han, Z. B.; Ding, K. L. Angew. Chem., Int. Ed. 2013, 52, 4744.
/
| 〈 |
|
〉 |