

酰胺类杂环烯酮缩胺的合成
收稿日期: 2016-07-02
修回日期: 2016-08-25
网络出版日期: 2016-09-08
基金资助
国家自然科学基金(Nos. 21362042,U1202221,21662042)、云南省后备人才(No. 2012HB001)、云南大学青年英才计划(No. XT412003)资助项目.
Synthesis of Amide Class Heterocyclic Ketene Aminals
Received date: 2016-07-02
Revised date: 2016-08-25
Online published: 2016-09-08
Supported by
Project supported by the National Natural Science Foundation of China(Nos. 21362042, U1202221, 21662042), the Talent Found in Yunnan Province(No. 2012HB001), and the Excellent Young Talents, Yunnan University(No. XT412003).
罗大云 , 崔时胜 , 胡兴梅 , 林军 , 严胜骄 . 酰胺类杂环烯酮缩胺的合成[J]. 有机化学, 2017 , 37(1) : 166 -175 . DOI: 10.6023/cjoc201607002
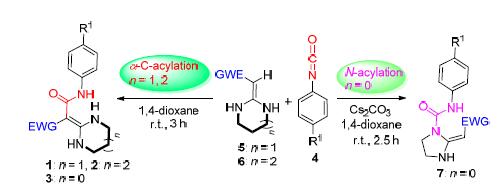
In this paper,a concise protocol for synthesis of possess potential pharmacological active amide class heterocyclic ketene aminals(HKAs) has been developed,which based on the six or seven-membered HKAs 1~2,using aryl isocyanate 4 as acylation agent.The regioselective acylation reaction of HKAs in 1,4-dioxane at room temperature gave compounds 5~6 with 90%~98% yields.However,the five-membered HKAs 3 and aryl isocyanate 4 reacted in 1,4-dioxane catalyzed by Cs2CO3 at room temperature to obtain compounds 7 in 78%~93% yields.
[1] (a) Batra, S.; Tusi, Z.; Madappaa, S. Curr. Med. Chem.:An-ti-Infect. Agents 2006, 5, 135.
(b) He, X.; Zhong, M.; Yang, J.; Wu, Z.; Xiao, Y.; Guo, H.; Hu, X. Chem. Biol. Drug Des. 2012, 79, 771.
(c) Li, H.-Q.; Zhu, T.-T.; Yan, T.; Luo, Y.; Zhu, H.-L. Eur. J. Med. Chem. 2009, 44, 453.
[2] (a) Nourry, A.; Zambon, A.; Davies, L.; Niculescu-Duvaz, I.; Dijkstra, H. P.; Ménard, D.; Gaulon, C.; Niculescu-Duvaz, D.; Suijkerbuijk, B. M.; Friedlos, F.; Manne, H. A.; Kirk, R.; Whittaker, S.; Marais, R.; Springer, C. J. J. Med. Chem. 2010, 53, 1964.
(b) Ménard, D.; Niculescu-Duvaz, I.; Dijkstra, H. P.; Niculescu-Duvaz, D.; Suijkerbuijk, B. M.; Zambon, A.; Nourry, A.; Roman, E.; Davies, L.; Manne, H. A.; Friedlos, F.; Kirk, R.; Whittaker, S.; Gill, A.; Taylor, R. D.; Marais, R.; Springer, C. J. J. Med. Chem. 2009, 52, 3881.
(c) Reddy, N. V.; Kumar, P. S.; Reddy, P. S.; Kantam, M. L.; Reddy, K. R. New J. Chem. 2015, 39, 805.
(d) Wang, W.-L.; Luo, H.; Gao, Y.; Gao, L.-X.; Cheng, L.; Zhou, Y.-B.; Li, J.; Li, J.-Y.; Feng, B.-N. 2016, 36, 2142(in Chinese).(王文龙, 骆欢, 高雅, 高立信, 盛丽, 周宇波, 李佳, 李静雅, 冯柏年, 有机化学, 2016, 36, 2142.)
[3] (a) Yu, S.; Haight, A.; Kotecki, B.; Wang, L.; Lukin, K.; Hill, D. H. J. Org. Chem. 2009, 74, 9539.
(b) Clayden, J.; Hennecke, U. Org. Lett. 2008, 10, 3567.
(c) Clayden, J.; Dufour, J.; Grainger, D. M.; Helliwell, M. J. Am. Chem. Soc. 2007, 129, 7488.
(d) Gao, J.; Li, H.; Zhang, Y.; Zhang, Y. Green Chem. 2007, 9, 572.
(e) Sheng, G..; Zhang, W.; Chin. J. Org. Chem. 2013, 33, 2271(in Chinese).(盛国柱, 张炜, 有机化学, 2013, 33, 2271.)
(f) Yang, R.; Zhao, Y.; Jiang, M.; Yan, S.; Lin, J. Chin. J. Org. Chem. 2016, 36, 2941(in Chinese).(杨瑞霞, 赵宇澄, 蒋美妤, 严胜骄, 林军, 有机化学, 2016, 36, 2941.)
[4] (a) Gallou, I. Org. Prep. Proced. Int. 2007, 4, 355.
(b) Bigi, F.;Maggi, R.; Sartori, G. Green Chem. 2000, 2, 140.
(c) Zhang, Z.; Schreiner, P. R. Chem. Soc. Rev. 2009, 38, 1187.
(d) Wang, H.; Zhao, W. Chin. J. Org. Chem. 2013, 33, 1822(in Chinese).(王宏社, 赵卫星, 有机化学, 2013, 33, 1822.)
[5] (a) Fabio, R. D.; Griffante, C.; Alvaro, G.; Pentassuglia, G.; Pizzi, D. A.; Donati, D.; Rossi, T.; Guercio, G.; Mattioli, M.; Cimarosti, Z.; Marchioro, C.; Provera, S.; Zonzini, L.; Montanari, D.; Melotto, S.; Gerrard, P. A.; Trist, D. G.; Ratti, E.; Corsi, M. J. Med. Chem. 2009, 52, 3238.
(b) Cao, P.; Huang, X.-F.; Ding, H.; Ge, H.-M.; Li, H.-Q.; Ruan, B.-F.; Zhu, H.-L. Chem. Biodiversity 2007, 4, 881.
(c) Debnath, A. K. J. Med. Chem.1999, 42, 249.
(d) Zhang, X.; Rodrigues, J.; Evans, L.; Hinkle, B.; Ballantyne, L.; Pena, M. J. Org. Chem. 1997, 62, 6420.
[6] Loto, R. T.; Loto, C. A.; Popoola, A. P. I. J. Mater. Environ. Sci. 2012, 3, 885.
[7] Bucha, H. C.; Todd, C. W. Science 1951, 114, 493.
[8] Bigi, F.; Maggi, R.; Sartori, G.; Zambonin, E. Chem. Commun. 1998, 4, 513.
[9] (a) Li, M.; Shao, P.; Wang, S.-W.; Kong, W.; Wen, L.-R. J. Org. Chem. 2012, 77, 8956.
(b) Yu, F.-C.; Huang, R.; Ni, X.-C.; Fan, J.; Yan, S.-J.; Lin, J. Green Chem. 2013, 15, 453.
(c) Yaqub, M.; Arif, N.; Perveen, R.; Batool1, J.; Riaz, M. T.; Yaseen, M. Asian J. Org. Chem. 2015, 27, 1013.
(d) Zhou, B.; Liu, Z.-C.; Qu, W.-W.; Yang, R.; Lin, X.-R.; Yan, S.-J.; Lin, J. Green Chem. 2014, 16, 4359.
(e) Xu, W.-Y.; Jia, Y.-M.; Yang, J.-K.; Huang, Z.-T.; Yu, C.-Y. Sylett 2010, 1682.
(f) Kong, L.; Yang, R.; Du, X.; Yan, S.; Lin, J. Chin. J. Org. Chem. 2016, 36, 1994(in Chinese).(孔令斌, 杨瑞霞, 杜璇璇, 严胜骄, 林军, 有机化学, 2016, 36, 1994.)
[10] (a) Wang, K.-M.; Yan, S.-J.; Lin, J. Eur. J. Chem. 2014, 1129.
(b) Zheng, C.-C.; Liu, F.-J.; Ping, D.-W.; Hu, L.-M.; Cai, Y.-L.; Zhong, R. G. J. Org. Chem. 2009, 74, 6386.
(c) Chen, X.-B.; Liu, Z.-C.; Yang, L.-F.; Yan, S.-J.; Lin, J. ACS Sustainable Chem. Eng. 2014, 2, 1155.
(d) Chen, X.-B.; Liu, Z.-C.; Lin, X.-R.; Huang, R.; Yan, S.-J.; Lin, J. ACS Sustainable Chem. Eng. 2014, 2, 2391.
(e) Peng, M.; Yang, R.; Liu, X.; Yan, S.; Lin, J. Chin. J. Org. Chem. 2015, 35, 1754(in Chinese).(彭美阳, 杨瑞霞, 刘昔敏, 严胜骄, 林军, 有机化学, 2015, 35, 1754.)
[11] (a) Yu, F.-C.; Yan, S.-J.; Hu, L.; Wang, Y.-C.; Lin, J. Org. Lett. 2011, 13, 4782.
(b) Yu, F.-C.; Hao, X.-P.; Lin, X.-R.; Yan, S.-J.; Lin, J. Tetra-hedron 2015, 71, 4084.
(c) Li, M.; Zhou, Z.-M.; Wen, L.-R.; Qiu, Z.-X. J. Org. Chem. 2011, 76, 3054.
(d) Wen, L.-R.; Li, Z.-R.; Li, M.; Cao, H. Green Chem. 2012, 14, 707.
(e) Wen, L.-R.; Sun, Q.-C.; Zhang, H.-L.; Li, M. Org. Biomol. Chem. 2013, 11, 781.
[12] (a) Huang, C.; Yan, S.-J.; Zeng, X.-H.; Dai, X.-Y.; Zhang, Y.; Qing, C.; Lin, J. Eur. J. Med. Chem. 2011, 46, 1172.
(b) Yan, S.-J.; Liu, Y.-J.; Chen, Y.-L.; Liu, L.; Lin, J. Bioorg. Med. Chem. Lett. 2010, 20, 5225.
(c) Yan, S.-J.; Huang, C.; Zeng, X.-H.; Huang, R.; Lin, J. Bioorg. Med. Chem. Lett. 2010, 20, 48.
[13] (a) Chen, N.;Meng, X.; Zhu, F.; Cheng, J.; Shao, X.; Li, Z. J. Agric. Food Chem. 2015, 63, 1360.
(b) Shao, X.; Fu, H.; Xu, X.; Xu, X.; Liu, Z.; Li, Z.; Qian, X. J. Agric. Food Chem. 2010, 58, 2696.
[14] Maryanoff, B. E.; Nortey, S. O.; McNally, J. J.; Sanfilippo, P. J.; McComsey, D. F.; Dubinsky, B.; Shank, R. P.; Reitz, A. B. Biomed. Chem. Lett. 1999, 9, 1547.
[15] Abdelhalim, M. M.; El-Saidi, M. M. T.; Rabie, S. T.; Elmegeed, G. A. Steroids 2007, 72, 459.
[16] Wang, M.-X.; Huang, Z.-T. J. Org. Chem. 1995, 60, 2807.
[17] (a) Huang, Z.-T.; Wang, J.-C.; Wang, L.-B. Synth. Commun. 1996, 26, 2285.
(b) Zhao, M.-X.; Wang, M.-X.; Hang, Z.-T. Acta Chim. Sinica 2001, 59, 1763(in Chinese).(赵梅欣, 王梅祥, 黄志镗, 化学学报, 2001, 59, 1763.)
(c) Rajappa, S.; Advani, B. G.; Sreenivasan, R. Indian J. Chem. 1977, 15(B), 886.
[18] Yu, C.-Y.; Yan, S.-J.; Zhang, T.; Huang, Z.-T. CN 101041660, 2007[Chem. Abstr. 2007, 147, 469361].
[19] Liu, B.; Wang, M.-X.; Huang, Z.-T. Synth. Commun. 1999, 29, 4241.
[20] Baum, K.; Nguyen, N. V.; Gilardi, R.; Flippen-Anderson, J. L.; George, C. J. Org. Chem. 1992, 57, 3026.
[21] Jiang, X.-Y.; Liu, Z.-C.; Fang, L.; Yang, S.-J.; Lin, J. RSC Adv. 2014, 4, 26389.
[22] (a) Wan, J.-P.; Gao, Y. Chem. Rec. 2016, 16, 1164.
(b) Cao, S.; Jing, Y.; Liu, Y.; Wan, J. Chin. J. Org. Chem. 2014, 34, 876(in Chinese).(曹硕, 景艳锋, 刘云云, 万结平, 有机化学, 2014, 34, 876.)
[23] Wan, J.-P.; Gan, S.-F.; Sun, G.-L.; Pan, Y.-J. J. Org. Chem. 2009, 74, 2862.
[24] Wan, J.-P.; Lin, Y.; Jing, Y.; Xu, M.; Liu, Y. Tetrahedron 2014, 70, 7874.
[25] Cao, S.; Xin, L.; Liu, Y.; Wan, J.-P.; Wen, C. RSC Adv. 2015, 5, 27372.
[26] (a) Huang, Z.-T.; Wang, M.-X. Synthesis 1992, 1273.
(b) Li, Z.-J.; Charles, D. Synth. Commun. 2001, 31, 527.
(c) Chen, X.-B.; Liu, X.-M.; Huang, R.; Yan, S. J.; Lin, J. Eur. J. Org. Chem. 2013, 4607.
/
| 〈 |
|
〉 |