

α-三氟甲基-α-羟基Weinreb酰胺的合成研究
收稿日期: 2016-06-22
修回日期: 2016-09-12
网络出版日期: 2016-09-19
基金资助
国家自然科学基金(Nos. 21262031,21462037)资助项目.
Synthesis of α-Trifluoromethyl-α-hydroxyl Weinreb Amides
Received date: 2016-06-22
Revised date: 2016-09-12
Online published: 2016-09-19
Supported by
Project supported by the National Natural Science Foundation of China(Nos. 21262031, 21462037).
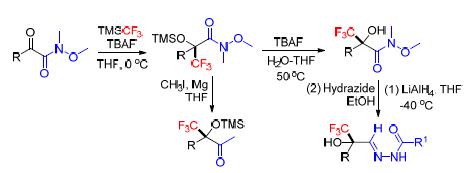
探索了三氟甲基三甲基硅烷与α-羰基Weinreb酰胺选择性三氟甲基化反应,合成了一系列α-三氟甲基-α-羟基Weinreb酰胺类化合物,并考察了其作为三氟甲基合成砌块与有机金属试剂的反应.
关键词: 三氟甲基化; 三氟甲基三甲基硅烷; α-三氟甲基-α-羟基Weinreb酰胺; 格氏试剂
康娟 , 黄丹凤 , 王克虎 , 苏瀛鹏 , 胡雨来 , 常青 . α-三氟甲基-α-羟基Weinreb酰胺的合成研究[J]. 有机化学, 2017 , 37(1) : 103 -109 . DOI: 10.6023/cjoc201606034
Selective trifluoromethylation of α-oxo-Weinreb amides was explored using trimethyl(trifluoromethyl) silane as fluorinating reagent.A series of α-trifluoromethyl-α-hydroxyl Weinreb amides were successfully synthesized and their reaction with organometallic reagents was investigated.

[1] Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
[2] Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; Pozo, C. D.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432.
[3] Ojima, I. Fluorine in Medicinal Chemistry and Chemical Biology, Wiley-Blackwell, Hong Kong, 2009.
[4] Cametti, M.; Crousse, B.; Metrangolo, P.; Milani, R.; Resnati, G. Chem. Soc. Rev. 2012, 41, 31.
[5] Littich R.; Scott, P. J. H. Angew. Chem., Int. Ed. 2012, 51, 1106.
[6] Chu, L.; Qing, F.-L. Acc. Chem. Res. 2014, 47, 1513.
[7] Liu, X.; Xu, C.; Wang, M.; Liu, Q. Chem. Rev. 2015, 115, 683.
[8] Ni, C.; Hu, M.; Hu, J. Chem. Rev. 2015, 115, 765.
[9] Alonso, C.; Marigorta, E. M.; Rubiales, G.; Palacios, F. Chem. Rev. 2015, 115, 1847
[10] Nahm S.; Weinreb, S. M. Tetrahedron Lett. 1981, 22, 3815.
[11] Zhao, W.; Liu, W. Chin. J. Org. Chem. 2015, 35, 55(in Chinese).(赵蔚, 刘伟, 有机化学, 2015, 35, 55.)
[12] Singh, J.; Satyamurthi, N.; Aidhen, I. S. J. Prakt. Chem./Chem-Ztg. 2000, 342, 340.
[13] Sibi, M. P. Org. Prep. Proced. Int, 1993, 25, 15.
[14] Pérez, M.; Pozo, C. D.; Reyes, F.; Rodríguez, A.; Francesch, A.; Echavarren, A. M.; Cuevas, C. Angew. Chem., Int. Ed. 2004, 43, 1724.
[15] Lygo, B.; Bhatias, M.; Cooke, J. W. B.; Hirst, D. J. Tetrahedron Lett. 2003, 44, 2529.
[16] Dias, L. C.; Sousa, M. A. D. Tetrahedron Lett. 2003, 44, 5625.
[17] Shimizu, T.; Kusada, J.; Ishiyama, H.; Nakata, T. Tetrahedron Lett. 2003, 44, 4965.
[18] Taillier, C.; Bellosta, V.; Meyer, C.; Cossy, J. Org. Lett. 2004, 6, 2145.
[19] Murphy, J. A.; Commeureuc, A. G. J.; Snaddon, T. N.; McGuire, T. M.; Khan, T. A.; Hisler, K.; Dewis, M. L.; Carling, R. Org. Lett. 2005, 7, 1427.
[20] Banks, R. E.; Smart, B. E.; Tatlow, J. C. Organofluorine Chemistry:Principles and Commercial Applications, Plenum, New York, 1994, p. 57.
[21] Quan, F.; Huang, D.; Xu, C.; Zhang, Z.; Niu, T.; Feng, H.; Cu, L.; HU, Y. Chin. J. Org. Chem. 2009, 29, 450(in Chinese).(权锋, 黄丹凤, 徐长明, 张正臣, 牛腾, 冯惠娟, 徐雷云, 胡雨来, 有机化学, 2009, 29, 450.)
[22] Wang, F.; Lu, A.; Huang, D.; Su, Y.; Xia, X.; Xu, Y.; Wang, K.-H.; Hu, Y. Chin. J. Org. Chem. 2015, 35, 1046(in Chinese).(王凤娇, 陆爱玲, 黄丹凤, 苏瀛鹏, 夏晓文, 徐艳丽, 王克虎, 胡雨来, 有机化学, 2015, 35, 1046.)
[23] Ma, l.; Lü, W.; Huang, D.; Niu, T.; Su, Y.; Wang, K.-H.; Hu, Y. Chin. J. Org. Chem. 2014, 34, 962(in Chinese).(马丽芳, 吕文贤, 黄丹凤, 牛腾, 苏瀛鹏, 王克虎, 胡雨来, 有机化学, 2014, 34, 962.)
[24] Prakash, G. K. S.; Krishnamurti, R.; Olah, G. A. J. Am. Chem. Soc. 1989, 111, 393.
[25] Stahly, G. P.; Bell, D. R. J. Org. Chem. 1989, 54, 2873.
[26] Singh, R. P.; Kirchmeier, R. L.; Shreeve, J. M. J. Org. Chem. 1999, 64, 2579.
[27] Sibi, M. P. Org. Prep. Proc. Int. 1993, 25, 15.
/
| 〈 |
|
〉 |