

对二茂铁苯甲酰噻二唑类化合物的合成和性质研究
收稿日期: 2016-07-05
修回日期: 2016-09-06
网络出版日期: 2016-09-19
基金资助
国家自然科学基金(No. 21171149)资助项目.
Synthesis and Properties of 4-Ferrocenyl-benzoyl-thiadiazole Derivatives
Received date: 2016-07-05
Revised date: 2016-09-06
Online published: 2016-09-19
Supported by
Project supported by the National Natural Science Foundation of China(No. 21171149).
任亚平 , 刘絮 , 王瑞 , 周元清 , 李标 , 徐琰 , 宋毛平 . 对二茂铁苯甲酰噻二唑类化合物的合成和性质研究[J]. 有机化学, 2017 , 37(1) : 110 -115 . DOI: 10.6023/cjoc201607008
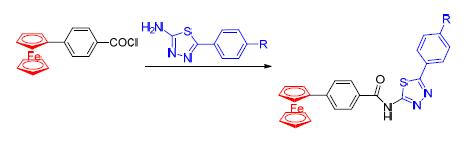
Seven kinds of ferrocenyl-benzoyl-thiadiazole compounds FcL1~FcL7 were synthesized by using 4-ferrocenyl-benzoic and 2-amino-5-aryl-1,3,4-thiadiazole as raw materials.These compounds were characterized by IR,1H NMR and elemental analysis.The crystal structure of FcL4 was determined by X-ray diffraction analysis.The electrochemical research showed that the redox reaction on the surface of electrode was reversible with single electron,and FcL1~FcL7 have a certain redox response to Pb2+ and Zn2+.The antibacterial activity tests indicated that FcL1~FcL7 presented significant activity and selectivity against Fusarium graminearum.FcL5~FcL7 exhibited good inhibition against human esophageal cancer cells in the anticancer activity tests.
Key words: ferrocene; thiadiazole; crystal structure; electrochemistry; biological activity
[1] El-Behairy, M. F.; Aboul-Enein, M. N; El-Azzouny, A. A. S.; Saleh, O. A.; Maklad, Y. A.; Aboutabl, M. E.; Maghraby, A. S.; Eur. J. Chem. 2014, 5, 488.
[2] Liu, T.-T.; Wan, Y.-C.; Fang, H. Chin. J. Org. Chem. 2016, 36, 417(in Chinese).(刘婷婷, 万义超, 方浩, 有机化学, 2016, 36, 417.)
[3] Yadagiri, B.; Gurrala, S.; Bantu, R.; Nagarapu, L.; Polepalli, S.; Srujana, G.; Jain, N. Bioorg. Med. Chem. Lett. 2015, 25, 2220.
[4] Yang, H.-W.; Guan, J.-J.; Gao, F.-X.; He, X.-P.; Wang, A.; Sun, D.-B.; Zhang, X.-Q.; Zhang, B.; Feng, Y.-Q. Chin. J. Org. Chem. 2015, 35, 129(in Chinese).(杨贺玮, 官俊杰, 高峰贤, 何欣平, 王安, 孙宝德, 张学强, 张宝, 冯亚青, 有机化学, 2015, 35, 129.)
[5] Joseph, A.; Shah, C. S.; Kumar, S. S.; Alex, A. T.; Maliyakkal, N.; Moorkoth, S. Acta Pharm. 2013, 63, 397.
[6] Megally Abdo, N. Y.; Kamel, M. M. Chem. Pharm. Bull. 2015, 63, 369.
[7] Suzuki, K.; Hamada, Y.; Nguyen, J. T.; Kiso, Y. Bioorg. Med. Chem. 2013, 21, 6665.
[8] Dekhane, D. V.; Pawar, S. S.; Gupta, S.; Shingare, M. S.; Patil, C. R.; Thore, S. N. Bioorg. Med. Chem. Lett. 2011, 21, 6527.
[9] Harish, K. P.; Mohana, K. N.; Mallesha, L.; Russ. J. Bioorg. Chem. 2014, 40, 97.
[10] Hu, Y.; Li, C.-Y.; Wang, X.-M.; Yang, Y.-H.; Zhu, H.-L. Chem. Rev. 2014, 114, 5572.
[11] Luo, Z.-H.; Chen, B.-Q.; He, S.-Y.; Shi, Y.-P.; Liu, Y.-M., Li, C.-W. Bioorg. Med. Chem. Lett. 2012, 22, 3191.
[12] Wu, K.-L.; Zhang, W.-B.; Zhou, D.; Xu, Y. Chin. J. Org. Chem. 2014, 34, 1201(in Chinese).(吴孔丽, 张吾斌, 周丹, 徐琰, 有机化学, 2014, 1201.)
[13] Liu, Y.-T.; Wang, J.; Yin, D.-W. Fine Chem. 2013, 30(in Chinese).(刘玉婷, 王捷, 尹大伟, 精细化工, 2013, 30.)
[14] Zhang, Q.; Zhao, B.; Song, Y.; Hua, C.; Gou, X.; Chen, B. Het-eroat. Chem. 2015, 26, 348.
[15] Pla?uk, D.; Zakrzewski, J.; Salmain, M.; B?au?, A.; Rychlik, B.; Strzelczyk, P. Organometallics 2013, 32, 5774.
[16] Chopra, R.; De Kock, C.; Smith, P.; Chibale, K.; Singh, K. Eur. J. Med. Chem. 2015, 100, 1.
[17] Caballero, A.; Lloveras, V.; Curiel, D.; Tárraga, A.; Espinosa, A.; García, R.; Veciana, J. Inorg. Chem. 2007, 46, 825.
[18] Gou, X.-F.; Li, Y.; Chen, B.; Gao, X.-Y. Chem. Reag. 2013, 35, 305(in Chinese).(苟小锋, 李媛, 陈邦, 高学祥, 化学试剂, 2013, 35, 305.)
[19] Yin, D.-W.; Sun, X.-M; Liu, Y.-T. Appl. Mech. Mater. 2012, 189, 181.
[20] Quintana, C.; Klahn, A. H.; Artigas, V.; Fuentealba, M.; Biot, C.; Halloum, I.; Halloum, I.; Kremer, L.; Arancibia, R. Inorg. Chem. Commun. 2015, 55, 48.
[21] Gu, L.-Q.; Li, J.; Fu, Y.-L.; Ran, C.-L.; Xu, Y.; Fan, Y.-T. Chin. J. Inorg. Chem. 2010, 23, 2113(in Chinese).(顾立强, 李杰, 傅亚丽, 冉春玲, 徐琰, 樊耀亭, 无机化学学报, 2010, 26, 2113.)
[22] Feng, Z.-J.; Wu, D.-L.; Shang, Y.-J. Chin. J. Synth. Chem. 2007, 15, 154(in Chinese).(冯志君, 吴德林, 商永嘉, 合成化学, 2007, 15, 154.)
[23] Xu, Y.; Zhu, L.-M.; Ran, C.-L.; Wang, H.-X.; Fan, Y.-T. Chin. J. Inorg. Chem. 2007, 23, 589(in Chinese).(徐琰, 朱丽敏, 冉春玲, 王海先, 樊耀亭, 无机化学学报, 2007, 23, 589.)
[24] Khan, N.; Badshah, A.; Lal, B.; Malik, M. A.; Raftery, J.; O'Brien, P.; Altaf, A. A. Polyhedron 2014, 69, 40.
[25] Nikalje, A. P. G.; Shaikh, S. I.; Khan, F. A. K.; Shaikh, S.; Sangshetti, J. N. Med. Chem. Res. 2015, 24, 4058.
/
| 〈 |
|
〉 |