

含脲基Schiff碱大环合成、结构及其对阴离子的识别研究
收稿日期: 2016-05-28
修回日期: 2016-09-02
网络出版日期: 2016-10-08
基金资助
国家自然科学基金(No.21061003)、教育部春晖计划(No.Z2012054)资助项目.
Synthesis, Structure and Anion Recognition of Urea-Functionalized Schiff Base Macrocyclic Compound
Received date: 2016-05-28
Revised date: 2016-09-02
Online published: 2016-10-08
Supported by
Project supported by the National Natural Science Foundation of China (No. 21061003), and the ‘Chun-Hui’ Fund of Ministry of Education (No. Z2012054).
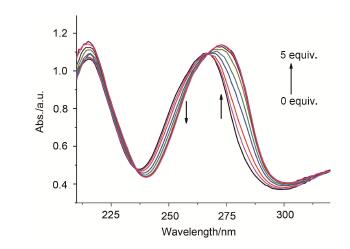
采用前体二胺1,3-二(3-胺基苯基)脲(1)和二醛1,3-二(2-甲酰基苯氧基)-2-丙醇(2)进行缩合作用得到[1+1] Schiff碱大环3.用1H NMR、FT-IR、FABMS和元素分析等对大环组成进行了表征,并通过X射线单晶衍射技术解析了大环3的晶体结构.采用UV-vis光谱滴定技术对大环与系列阴离子的键合作用进行了考察,结果表明,Schiff碱大环3对AcO-离子有明显的选择性识别作用,并用紫外-可见吸收光谱、核磁和等温量热滴定等技术分别对大环3与AcO-阴离子的识别反应进行了考察,获得了识别反应的配位比、平衡常数(K)及ΔrHm、ΔrSm、ΔrGm等热力学参数信息.
张文龙 , 陈冬梅 , 刘兴丽 , 黄超 , 朱必学 . 含脲基Schiff碱大环合成、结构及其对阴离子的识别研究[J]. 有机化学, 2017 , 37(2) : 474 -479 . DOI: 10.6023/cjoc201605042
A novel [1+1] Schiff base macrocyclic compound 3 has been synthesized from the reaction of precursors 1,3-bis(3-amino-phenyl)-urea (1) with 1,3-bis(2-formylphenoxy)-2-propa-nol (2). The macrocycle 3 was characterized by 1H NMR, FT-IR, FABMS spectra and elemental analysis, and the crystal structure of 3 was determined by X-ray diffraction analy-sis. The results show that the macrocycle 3 displays a selective recognition ability for AcO- ion by the coordination reaction of 3 with a series of anions using UV-vis absorption spectra technique. Furthermore, the coordination reaction of 3 with AcO- ion was investigated via UV-Vis spectra, 1H NMR and the isothermal titration calorimeter (ITC) respectively. The stoichiometric ratio, the association constant (K) and the thermodynamic parameters (ΔrHm, ΔrSm and ΔrGm) of the coordination reaction were obtained.

Key words: Schiff base macrocycle; crystal structure; Urea; AcO-; recognition
[1] Beer, P. D.; Gale, P. A. Angew. Chem., Int. Ed. 2001, 40, 486.
[2] Martinez-Manez, R.; Sancenon, F. Chem. Rev. 2003, 103, 4419.
[3] Li, A. F.; Wang, J. H.; Wang, F.; Jiang, Y. B. Chem. Soc. Rev. 2010, 39, 3729.
[4] Li, C. T.; Cao, Q. Y.; Li, J. J.; Wang, Z. W.; Dai, B. N. Inorg. Chim. Acta 2016, 449, 31.
[5] Wang, Z.-C.; Dai, B.-N.; Qiu, J.-F.; Cao, Q.-Y.; Ge, J.-Z. Chin. J. Org. Chem. 2015, 35, 2383 (in Chinese).(王智成, 戴博娜, 丘继芳, 曹迁永, 葛金柱, 有机化学, 2015, 35, 2383.)
[6] Gale, P. A. Acc. Chem. Res. 2006, 39, 465.
[7] Huang, Z.-B.; Liu, X.-C.; Hu, M.-H.; Lin, W.; Shi, D.-Q. Chin. J. Org. Chem. 2014, 34, 382 (in Chinese).(黄志斌, 刘学成, 胡明华, 林伟, 史达清, 有机化学, 2014, 34, 382.)
[8] Wenzel, M.; Hiscock, J. R.; Gale, P. A. Chem. Soc. Rev. 2012, 41, 480.
[9] Zhang, F.; Tan, Z.; Yan, B.-R.; Pan, D.-W.; Bao, X.-P. Chin. J. Org. Chem. 2014, 34, 2499 (in Chinese).(张峰, 谭赞, 闫柏任, 潘顶伍, 鲍小平, 有机化学, 2014, 34, 2499.)
[10] Du, J.; Kang, K.; Hu, J. C.; Mao, L. J.; Yuan, L. H.; Feng, W. Chin. J. Chem. 2016, 34, 1.
[11] Amendola, V.; Fabbrizzi, L.; Mosca, L. Chem. Soc. Rev. 2010, 39, 3889.
[12] Boiocchi, M.; Del, B. L.; Gómez, D. E.; Fabbrizzi, L.; Licchelli, M.; Monzani, E. J. Am. Chem. Soc. 2004, 126, 16507.
[13] Jian, J.-Y.; Yan, B.-R.; Pan, D.-W.; Tan, Z.; Lv, X.-Y.; Du, H.; Bao, X.-P. Chin. J. Org. Chem. 2015, 36, 1069 (in Chinese).(蹇军友, 闫柏任, 潘顶伍, 谭赞, 吕新阳, 杜欢, 鲍小平, 有机化学, 2015, 36, 1069.)
[14] Chutia, R.; Dey, S. K.; Das, G. Cryst. Growth Des. 2013, 13, 883.
[15] Zhou, Y.; Zhang, J. F.; Yoon, J. Chem. Rev. 2014, 114, 5511.
[16] Srikala, P.; Tarafder, K.; Trivedi, D. R. Spectrochim. Acta, Part A 2017, 170, 29.
[17] Yan, X.-Q.; Zhuo, J.-B.; Wang, J.-C.; Xie, L.-L.; Yuan, Y.-F. Chin. J. Org. Chem. 2015, 35, 2184 (in Chinese).(晏希泉, 卓继斌, 王吉成, 谢莉莉, 袁耀锋, 有机化学, 2015, 35, 2184.)
[18] Özkar, S.; Ülkü, D.; Y?ld?r?m, L. T.; Biricik, N.; Gümgüm, B. J. Mol. Struct. 2004, 688, 207.
[19] Ou, M.; Den, Y.-X.; Wang, F.-F.; Zhu, C.; Zhang, Q.-L.; Zhu, B.-X. Chin. J. Org. Chem. 2013, 33, 1798 (in Chinese).(欧敏, 邓雅欣, 王芳芳, 朱纯, 张奇龙, 朱必学, 有机化学, 2013, 33, 1798.)
[20] Li, Q.; Zhang, Y. Q.; Zhu, Q. J.; Xue, S. F.; Xiao. X.; Tao, Z. Chem.-Asian J. 2015, 10, 1159.
[21] Boiocchi, M.; Colasson, B.; Fabbrizzi, L.; Monti, E. Inorg. Chim. Acta 2007, 360, 1163.
[22] Khandar, A. A.; Hosseini-Yazdi, S. A. Polyhedron 2003, 22, 1481.
[23] Sheldrick, G. M. SHELX-97, University of Göttingen, Germany, 1997.
/
| 〈 |
|
〉 |