

温和条件下去丙酮保护制备末端单炔烃及末端二炔的方法
收稿日期: 2016-07-20
修回日期: 2016-09-19
网络出版日期: 2016-10-11
基金资助
国家自然科学基金(No.21202050)、湖北省自然科学基金(No.2014CFB930)资助项目.
Synthesis of Terminal Alkynes/Diynes through Deprotection of Acetone Protected Alkynes under Mild Conditions
Received date: 2016-07-20
Revised date: 2016-09-19
Online published: 2016-10-11
Supported by
Project supported by the National Natural Science Foundation of China (No. 21202050), and the Natural Science Foundation of Hubei Province (No. 2014CFB930).
末端炔烃是有机合成中重要的原料或中间体.通过钯催化的Sonogashira偶联反应,将2-甲基-3-丁炔-2-醇连接到其它基团上所得到的结构,可以看作是一类丙酮保护的末端炔烃.目前,对于这种丙酮保护的末端炔烃,其去保护得到端基炔主要是通过KOH存在下在甲苯中回流数小时的方法,但是反应温度高,条件苛刻,适用范围较窄.对这一脱保护过程进行研究,通过对多种溶剂和反应温度进行对比,发现以1,4-二氧六环作为溶剂,KOH作为碱,可以在相对较低的温度(60℃)下得到较高的产率,而且所需时间大大缩短(0.5~2 h),拓展了这种方法的应用范围.
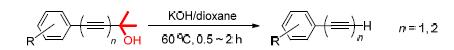
关键词: 1,4-二氧六环; 去保护; 末端炔烃; 2-甲基-3-丁炔-2-醇
关志朋 , 师瑶 , 石炜 , 陈浩 . 温和条件下去丙酮保护制备末端单炔烃及末端二炔的方法[J]. 有机化学, 2017 , 37(2) : 418 -422 . DOI: 10.6023/cjoc201607032
Terminal alkynes are important building blocks in organic synthesis. Acetone protected terminal alkynes are frequently used in alkyne synthesis and reactions, which could be easily prepared by Pd-catalyzed Sonogashira cross-coupling reactions between electrophiles and 2-methyl-3-butyn-2-ol. At present, the deprotection of the acetone protected terminal alkynes is generally carried out in reflux toluene in the presence of potassium hydroxide for hours. However, this procedure suffers from the harsh reaction conditions and its application has been limited. Here in the deprotection of acetones has been discussed, and various solvent, temperature, substrates were investigated to advance the procedure, and it is found that by employing 1,4-dioxane as solvent, this process can undergo smoothly at 60℃ with satisfying yields in 0.5~2 h in the presence of 2.0 equiv. KOH.
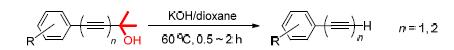
Key words: 1,4-dioxane; deprotection; terminal alkyne; 2-methyl-3-butyn-2-ol
[1] Siemsen, P.; Livingston, R. C.; Diederich, F. Angew. Chem., Int. Ed. 2000, 39, 2632.
[2] Shun, A. L. K. S.; Tykwinski, R. R. Angew. Chem., Int. Ed. 2006, 45, 1034.
[3] Ross, C.; Scherlach, K.; Kloss, F.; Hertweck, C. Angew. Chem., Int. Ed. 2014, 53, 7794.
[4] Tykwinski, R. R.; Chalifoux, W.; Eisler, S.; Lucotti, A.; Tommasini, M.; Fazzi, D.; Del, Z. M.; Zerbi, G. Pure Appl. Chem. 2010, 82, 891.
[5] Chalifoux, W. A.; Tykwinski, R. R. Nat. Chem. 2010, 2, 967.
[6] Shi, W. Curr. Organocatal. 2015, 2, 2.
[7] Tykwinski, R. R.; Chalifoux, W.; Eisler, S.; Lucotti, A.; Tommasini, M.; Fazzi, D.; Del Zoppo, M.; Zerbi, G. Pure Appl. Chem. 2010, 82, 891.
[8] Shi, W.; Lei, A. Tetrahedron Lett. 2014, 55, 2763.
[9] Stefko, M.; Tzirakis, M. D.; Breiten, B.; Ebert, M.-O.; Dumele, O.; Schweizer, W. B.; Gisselbrecht, J.-P.; Boudon, C.; Beels, M. T.; Biaggio, I.; Diederich, F. Chem.-Eur. J. 2013, 19, 12693.
[10] Sahnoune, H.; Baranova, Z.; Bhuvanesh, N.; Gladysz, J. A.; Halet, J.-F. Organometallics 2013, 32, 6360.
[11] Bai, D.; Li, C.; Li, J.; Jia, X. Chin. J. Org. Chem. 2012, 32, 994 (in Chinese).(白东虎, 李春举, 李健, 贾学顺, 有机化学, 2012, 32, 994.)
[12] Wang, Y.-F.; Deng, W.; Liu, L.; Guo, Q.-X. Chin. J. Org. Chem. 2005, 25, 8 (in Chinese).(王晔峰, 邓维, 刘磊, 郭庆祥, 有机化学, 2005, 25, 8.)
[13] Shi, W.; Luo, Y. D.; Luo, X. C.; Chao, L.; Zhang, H.; Wang, J.; Lei, A. W. J. Am. Chem. Soc. 2008, 130, 14713.
[14] Zhang, F.-G.; Ma, H.; Zheng, Y.; Ma, J.-A. Tetrahedron 2012, 68, 7663.
[15] Xue, Y.-X.; Zhu, Y.-Y.; Gao, L.-M.; He, X.-Y.; Liu, N.; Zhang, W.-Y.; Yin, J.; Ding, Y.; Zhou, H.; Wu, Z.-Q. J. Am. Chem. Soc. 2014, 136, 4706.
[16] Carpita, A.; Mannocci, L.; Rossi, R. Eur. J. Org. Chem. 2005, 1859.
[17] Li, S.; Mao, F.; Shi, Y.; Wang, Y.; Shi, W. J. Fujian Normal Univ. (Nat. Sci.) 2015, 31, 120 (in Chinese).(李硕, 毛凤, 师瑶, 王阳, 石炜, 福建师范大学学报(自然科学版), 2015, 31, 120.)
[18] Jiang, M. X.-W.; Rawat, M.; Wulff, W. D. J. Am. Chem. Soc. 2004, 126, 5970.
[19] Havens, S. J.; Hergenrother, P. M. J. Org. Chem. 1985, 50, 1763.
[20] Danilkina, N. A.; Kulyashova, A. E.; Khlebnikov, A. F.; Bräse, S.; Balova, I. A. J. Org. Chem. 2014, 79, 9018.
[21] West, K.; Wang, C.; Batsanov, A. S.; Bryce, M. R. J. Org. Chem. 2006, 71, 8541.
[22] Li, J.; Huang, P. Beilstein J. Org. Chem. 2011, 7, 426.
[23] Li, J.; Guo, K.; Dong, Q.; Huang, P.; Wang, H.; Xu, B. CN 104177209, 2014 [Chem. Abstr. 2014, 2020360].
[24] Kabalka, G. W.; Zhou, L.-L.; Wang, L.; Pagni, R. M. Tetrahedron 2006, 62, 857.
[25] Ghaffarzadeh, M.; Bolourtchian, M.; Fard, Z. H.; Halvagar, M. R.; Mohsenzadeh, F. Synth. Commun. 2006, 36, 1973.
[26] Vaughn, T. H.; Nieuwland, J. A. J. Am. Chem. Soc. 1934, 56, 1207.
/
| 〈 |
|
〉 |