

一种荧光增强的反应型硫化氢荧光探针
收稿日期: 2016-07-20
修回日期: 2016-10-08
网络出版日期: 2016-10-18
基金资助
国家自然科学基金(No. 21233011)和国家重点基础研究发展计划(973计划,Nos. 2013CB834703,2013CB834505)资助项目.
A Reaction-Based Chemsensor for Hydrogen Sulfide Detection with Fluorescence Enhancement
Received date: 2016-07-20
Revised date: 2016-10-08
Online published: 2016-10-18
Supported by
Project supported by the the National Natural Science Foundation of China(No. 21233011) and the State Key Development Program for Basic Research of China(973 Program, Nos. 2013CB834703, 2013CB834505).
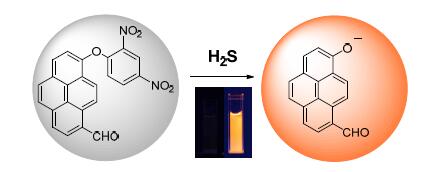
基于二硝基苯醚的硫解反应识别机制,设计合成了羟基芘甲醛为荧光基团的探针分子8-(2,4-二硝基苯酚基)芘甲醛(PCNP),研究了PCNP缓冲溶液分散体系对H2S的响应.未与H2S作用时,分子内光致电子转移过程导致探针分子PCNP几乎不发光,当体系中存在H2S时,PCNP发生硫解反应,光致电子转移过程被阻断,羟基芘甲醛发出橙色荧光.PCNP分子对H2S响应迅速、灵敏,0.1 mmol·L-1硫化氢存在下10 min内荧光强度响应达到最大值,荧光增强达260倍,反应速率常数为0.20 min-1,探针分子对H2S检测限为0.10 μmol·L-1,并且具有良好选择性.
周婵 , 邱波 , 曾毅 , 陈金平 , 于天君 , 李嫕 . 一种荧光增强的反应型硫化氢荧光探针[J]. 有机化学, 2017 , 37(1) : 92 -96 . DOI: 10.6023/cjoc201607034
A reaction-based probe 8-(2,4-dinitrophenoxy) pyrene-1-carbaldehyde(PCNP) for H2S detection was designed and synthesized by utilizing 8-hydroxypyrene-1-carbaldehyde and dinitrophenyl ether as the reporting chromophore and the recognition unit,respectively.The probe was dissolved in phosphate buffer saline(PBS) with cetyltrimethyl ammonium bromide(CTAB),and the sensing ability toward H2S was investigated with steady-state spectroscopy.The emission of PCNP was quenched by photoinduced electron transfer without H2S.The fluorescence of PCNP was boosted after thiolysis reaction in the presence of H2S,showing orange red emission.The fluorescence response was rapid and reached a maximum enhancement of about 260 times in the presence of 0.1 mmol·L-1 H2S,giving a reaction rate constant of 0.20 min-1.The limit of detection of PCNP is estimated to be 0.10 μmol·L-1.The results demonstrate that PCNP is capable of detecting H2S rapidly,sensitively and selectively.

[1] Lin, V. S.; Chen, W.; Xian, M.; Chang, C. J. Chem. Soc. Rev. 2015, 44, 4596.
[2] Kimura, H. Antioxid. Redox Signaling 2014, 20, 783.
[3] Paul, B. D.; Sbodio, J. I.; Xu, R. S.; Vandiver, M. S.; Cha, J. Y.; Snowman, A. M.; Snyder, S. H. Nature 2014, 509, 96.
[4] Hu, L. F.; Lu, M.; Tiong, C. X.; Dawe, G. S.; Hu, G.; Bian, J. S. Aging Cell 2010, 9, 135.
[5] Giuliani, D.; Ottani, A.; Zaffe, D.; Galantucci, M.; Strinati, F.; Lodi, R.; Guarini, S. Neurobiol. Learn. Mem. 2013, 104, 82.
[6] Gao, M.; Yu, F. B.; Chen, L. X. Prog. Chem. 2014, 26, 1065(in Chinese).(高敏, 于法标, 陈令新, 化学进展, 2014, 26, 1065.)
[7] Qian, Y.; Karpus, J.; Kabil, O.; Zhang, S. Y.; Zhu, H. L.; Banerjee, R.; Zhao, J.; He, C. Nat. Commun. 2011, 2, 495.
[8] Peng, B.; Chen, W.; Liu, C. R.; Rosser, E. W.; Pacheco, A.; Zhao, Y.; Aguilar, H. C.; Xian, M. Chem.-Eur. J. 2014, 20, 1010.
[9] Yu, H. B.; Li, H. L.; Zhang, X. F.; Xiao, Y.; Fang, P. J.; Lv, C. J.; Hou, W. Acta Chim. Sinica 2015, 73, 450(in Chinese).(于海波, 李红玲, 张新福, 肖义, 方沛菊, 吕春娇, 侯伟, 化学学报, 2015, 73, 450.)
[10] Zhao, C. C.; Zhang, X. L.; Li, K. B.; Zhu, S. J.; Guo, Z. Q.; Zhang, L. L.; Wang, F. Y.; Fei, Q.; Luo, S. H.; Shi, P.; Tian, H.; Zhu, W. H. J. Am. Chem. Soc. 2015, 137, 8490.
[11] Lippert, A. R.; New, E. J.; Chang, C. J. J. Am. Chem. Soc. 2011, 133, 10078.
[12] Zheng, Y.; Zhao, M.; Qiao, Q. L.; Liu, H. Y.; Lang, H. J.; Xu, Z. C. Dyes Pigm. 2013, 98, 367.
[13] Sun, W.; Fan, J. L.; Hu, C.; Cao, J. F.; Zhang, H.; Xiong, X. Q.; Wang, J. Y.; Cui, S.; Sun, S. G.; Peng, X. J. Chem. Commun. 2013, 49, 3890.
[14] Fan, F. L.; Jing, J. Q.; Chen, X. M. Chin. J. Org. Chem. 2014, 34, 2178(in Chinese).(范方禄, 靖金球, 陈雪梅, 有机化学, 2014, 34, 2178.)
[15] Sasakura, K.; Hanaoka, K.; Shibuya, N.; Mikami, Y.; Kimura, Y.; Komatsu, T.; Ueno, T.; Terai, T.; Kimura, H.; Naganot, T. J. Am. Chem. Soc. 2011, 133, 18003.
[16] Qu, X. Y.; Li, C. J.; Chen, H. C.; Mack, J.; Guo, Z. J.; Shen, Z. Chem. Commun. 2013, 49, 7510.
[17] Liu, J.; Guo, X. D.; Hu, R.; Liu, X. Y.; Wang, S. Q.; Li, S. Y.; Li, Y.; Yang, G. Q. Anal. Chem. 2016, 88, 1052.
[18] Fridkin, M.; Hazum, E.; Tauberfinkelstein, M.; Shaltiel, S. Arch. Biochem. Biophys. 1977, 178, 517.
[19] Liu, T. Y.; Xu, Z. C.; Spring, D. R.; Cui, J. N. Org. Lett. 2013, 15, 2310.
[20] Yuan, L.; Zuo, Q. P. Sens. Actuators, B 2014, 196, 151.
[21] Liu, Y.; Feng, G. Q. Org. Biomol. Chem. 2014, 12, 438.
[22] Cao, L. X.; Li, X. Y.; Wang, S. Q.; Li, S. Y.; Li, Y.; Yang, G. Q. Chem. Commun. 2014, 50, 8787.
[23] Qiu, B.; Zeng, Y.; Cao, L. X.; Hu, R.; Zhang, X. H.; Yu, T. J.; Chen, J. P.; Yang, G. Q.; Li, Y. RSC Adv. 2016, 6, 49158.
/
| 〈 |
|
〉 |