

C-2苯环上取代基对喹唑啉-4-酮烷基化的影响及化合物抗肿瘤、抗菌活性
收稿日期: 2016-08-31
修回日期: 2016-10-08
网络出版日期: 2016-11-03
基金资助
国家自然科学基金(No.21372060)及河北省自然科学杰出青年基金(培育)(No.B2015201005)资助项目.
Effects of the Substituent at C-2 Phenyl on the N-/O-Alkylation of Quinazolin-4(3H)-one and Anti-Tumor, Antimicrobial Activities of Some Compounds
Received date: 2016-08-31
Revised date: 2016-10-08
Online published: 2016-11-03
Supported by
Project supported by the National Natural Science Foundation of China (No. 21372060) and the Natural Science Fund for Distinguished Young Scholars of Hebei Province (Incubation) (No. B2015201005).
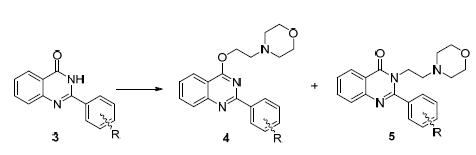
邻氨基苯甲酰胺与取代苯甲醛反应,合成了含氨烷基侧链的喹唑啉-4-酮4和5,探讨了C-2位苯环取代基对喹唑啉-4-酮内酰胺N-/O-烷基化的影响,并评价了部分化合物的抗肿瘤细胞增殖活性及抑菌活性.结果表明,当C-2苯环上的取代基在邻位时,N-烷基化反应为主;而在间位或对位时,以O-烷基化为主,立体效应起到了主导作用.4-{2-{{2-[3-(苄氧基)苯基]喹唑啉-4-基}氧基}乙基}吗啉(4h)具有较好的抗肺癌细胞增殖活性,IC50值为13.20 μmol/L.2-(2-氯苯基)-3-[2-(哌啶-1-基)乙基]喹唑啉-4(3H)-酮(5aa)和2-[3-(苄氧基)苯基]-4-[2-(吡咯啉-1-基)乙氧基]喹唑啉(4hb)(50 μg/mL)对大肠杆菌和痢疾杆菌具有显著的抑制活性,抑菌率分别为100%,100%和100%,96%.化合物5aa对链铬孢菌真菌的抑制率为100%.
王淑霞 , 高梦颖 , 谭官海 , 马海霞 , 赵莹莹 , 杜鸿源 , 王总帅 , 陈华 , 李小六 . C-2苯环上取代基对喹唑啉-4-酮烷基化的影响及化合物抗肿瘤、抗菌活性[J]. 有机化学, 2017 , 37(2) : 385 -393 . DOI: 10.6023/cjoc201608031
A series of quinazolin-4(3H)-one derivatives 4 and 5 possessing amino alkyl side chain were synthesized by the condensation reaction of 2-aminobenzamide with substituted benzaldehyde, followed by SN2 substitution reaction with haloalkane. The effects of the substituent at C-2 phenyl on the N-/O-alkylated reaction of quinazolin-4(3H)-one were explored. Some compounds were also evaluated for their anti proliferation activities and antimicrobial activities. The results showed that when the substituent was at orth O-position on C-2 phenyl, N-alkylation was the main reaction, while at meta- or para-positions, O-alkylation was predominant, which suggested that the steric factor played a key role on this ambident nuclophilic substitution. 4-(2-((2-(3-(Benzyloxy)phenyl)quinazolin-4-yl)oxy)ethyl)morpholine (4h) showed relatively good anti-proliferative activity against A549 tumor cells with the IC50 value of 13.20 μmol/L. 2-(2-Chlorophenyl)-3-(2-(piperidin-1-yl)ethyl)quinazolin-4(3H)-one (5aa) and 2-(3-(benzyloxy)phenyl)-4-(2-(pyrrolidin-1-yl)ethoxy)quinazoline (4hb) exhibited significant anti Esche-richia coli or Shigella castellani activities, and the inhibition rates were near 100% at the concentration of 50 μg/mL. The inhi-bition rate of compound 5aa against Alternaria alternate was 100%.

[1] Khan, I.; Zaib, S.; Batool, S.; Abbas, N.; Ashraf, Z.; Iqbal, Jamshed.; Saeed, A. Bioorg. Med. Chem. 2016, 24, 2361.
[2] Khan, I.; Ibrar, A.; Abbas, N.; Saeed, A. Eur. J. Med. Chem. 2014, 76, 193.
[3] Yan, B. R.; Lv, X. Y.; Du, H.; Bao, X. P. Chin. J. Org. Chem. 2016, 36, 207 (in Chinese).(闫柏任, 吕新阳, 杜欢, 鲍小平, 有机化学, 2016, 36, 207.)
[4] Ou, J. J.; Liu, K. C.; Wang, Y.; Zhang, H.; Liu, R. Q.; Li, Q. B.; Wang, Q. M.; Li, Y. Q.; Rui, C. H.; Liu, S. Z. Chin. J. Org. Chem. 2014, 34, 526 (in Chinese).(欧俊军, 刘克昌, 王毅, 张浩, 刘瑞全, 李奇博, 汪清民, 李永强, 芮昌辉, 刘尚钟, 有机化学, 2014, 34, 526.)
[5] Xu, W.; Jin, Y. B.; Liu, H. X.; Jiang, Y. Y.; Fu, H. Org. Lett. 2011, 13, 1274.
[6] Liu, C. E.; Yu, Q. Y.; Tang, J. H.; Li, J. R. Chin. J. Org. Chem. 2012, 32, 532 (in Chinese).(刘长娥, 于琪瑶, 唐健红, 李加荣, 有机化学, 2012, 32, 532.)
[7] Khan, I.; Ibrar, A.; Ahmed, W.; Saeed, A. Eur. J. Med. Chem. 2015, 90, 124.
[8] Usifoh, C. O.; Scriba, G. K. E. Pharm. Med. Chem. 2000, 333, 261.
[9] Hori, M.; Ohtaka, H. Chem. Pharm. Bull. 1993, 41, 1114.
[10] Chen, G. S.; Kalchar, S.; Kuo, C.-W.; Chang, C.-S.; Usifoh, C. O.; Chern, J.-W. J. Org. Chem. 2003, 68, 2502.
[11] Bogentoft, C.; Krongberg, L.; Danielsson, B. Acta Pharm. Suec. 1969, 6, 489.
[12] Chen, H.; Li, S.; Yao, Y. C.; Zhou, L. K.; Zhao, J. P.; Gu, Y. J.; Wang, K. R.; Li, X. L. Bioorg. Med. Chem. Lett. 2013, 23, 4785.
[13] Wang, Z. Z.; Tang, Y. Tetrahedron 2016, 72, 1330.
[14] Kim, N. Y.; Cheon, C.-H. Tetrahedron Lett. 2014, 55, 2340.
[15] Roopan, S. M.; Maiyalagan, T.; Khan, F. N. Can. J. Chem. 2008, 86, 1019.
[16] Shang, Y. H.; Fan, L. Y.; Li, X. X.; Liu, M. X. Chin. Chem. Lett. 2015, 26, 1355.
[17] Adib, M.; Ansari, S.; Mohammadi, A.; Bijanzadeh, H. R. Tetrahedron Lett. 2010, 51, 30.
[18] Zhan, D.; Li, T. B.; Zhang, X. P.; Dai, C.; Wei, H. H.; Zhang, Y. Y.; Zeng, Q. L. Synth. Commun. 2013, 43, 2493.
[19] Mosmann, T. J. Immunol. Methods 1983, 65, 55.
/
| 〈 |
|
〉 |