

N-(4-叔丁基-5-苄基噻唑-2-基)氨基乙酰胺的合成与抗肿瘤活性
收稿日期: 2016-10-12
修回日期: 2016-11-16
网络出版日期: 2016-11-29
基金资助
国家自然科学基金(No.21442014)资助项目.
Synthesis and Antitumor Activity of N-[4-(t-Butyl)-5-benzylthiazol-2-yl]amininoacetamides
Received date: 2016-10-12
Revised date: 2016-11-16
Online published: 2016-11-29
Supported by
Project supported by the National Natural Science Foundation of China (No. 21442014).
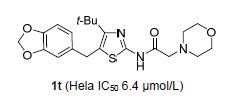
采用衍生法,在N-[4-叔丁基-5-(4-氯苄基)噻唑-2-基]脂肪酰胺酰基的α-位,插入氨基,设计合成了N-(4-叔丁基-5-苄基噻唑-2-基)氨基乙酰胺衍生物.以4,4-二甲基-1-芳基-3-戊酮为原料,经4-叔丁基-5-苄基-2-氨基噻唑(3),再经氯乙酰化和取代反应得21个N-(4-叔丁基-5-苄基噻唑-2-基)氨基乙酰胺(1),其结构经1H NMR、13C NMR和元素分析确证.噻唑蓝(MTT)法体外抗肿瘤活性测试表明,该类新化合物对肺癌细胞(A549)、宫颈癌细胞(Hela)和乳腺癌细胞(MCF-7)具有抗肿瘤活性.其中N-[4-叔丁基-5-(胡椒基)噻唑-2-基]吗啉基乙酰胺(1t)对Hela细胞的抑制活性最好;对优选化合物1t进行了AO/EB双染和细胞周期实验,流式细胞仪分析表明,化合物1t可显著诱导肿瘤细胞凋亡,阻滞Hela细胞有丝分裂在S期.
唐玉婷 , 丁娜 , 伍智林 , 叶姣 , 申坤 , 胡艾希 . N-(4-叔丁基-5-苄基噻唑-2-基)氨基乙酰胺的合成与抗肿瘤活性[J]. 有机化学, 2017 , 37(3) : 675 -682 . DOI: 10.6023/cjoc201610018
The antitumor activity research of N-(thiazol-2-yl) amide derivatives has been the focus of scholars. Physiologi-cally active substances containing amines widely exist in nature. A series of novel N-(4-(t-butyl)-5-benzylthiazol-2-yl) aminoacetamides were synthesized, and their structures were confirmed by 1H NMR, 13C NMR and elemental analysis. These compounds were evaluated for their in vitro anticancer activity on A549, Hela and MCF-7 cells. Most of the investigated compounds exhibited broad-spectrum antitumor activity. Compound 1t displayed significant activity against Hela cancer cells with IC50 value of (6.4±2.2) μmol/L. The AO/EB staining and cell cycle experiments were carried out on the preferred com-pound 1t. The result showed that compound 1t could significantly induce apoptosis of tumor cells and arrest the Hela cells in the S phase.

Key words: thiazoleacetamide; morpholine; synthesis; antitumor activity
[1] Pokhodylo, N.; Shyyka, O.; Matiychuk, V. Med. Chem. Res. 2014, 23, 2426.
[2] Lee, Y. S. E.; Chuang, S. H.; Huang, L. Y. L.; Lai, C. L.; Lin, Y. H.; Yang, J. Y.; Liu, C. W.; Yang, S. C.; Lin, H. S.; Chang, C. C.; Lai, J. Y.; Jian, P. S.; Lam, K.; Chang, J. M.; Lau, J. Y. N.; Huang, J. J. J. Med. Chem. 2014, 57, 4098.
[3] Gorczynski, M. J.; Leal, R. M.; Mooberry, S. L.; Bushweller, J. H.; Brown, M. L. Bioorg. Med. Chem. 2004, 12, 1029.
[4] Jiang, F. C.; Cheng, C. Y. Acta Pharm. Sin. 2006, 41, 727 (in Chinese).(姜凤超, 成冲云, 药学学报, 2006, 41, 727.)
[5] Bjoern, E.; Guido, K.; Christian, H. CN 101277692, 2007[Chem. Abstr. 2007, 146, 163101].
[6] Chen, Y.; Wang, H.; Dinesh, A.; Zhou, C. H. Chin. J. Org. Chem. 2016, 36, 1 (in Chinese).(程宇, 王辉, Dinesh Addla, 周成合, 有机化学, 2016, 36, 1.)
[7] Liao, Q. H.; Lin, S.; Deng, R. H.; Huang, Z. Q.; Deng, K. Y.; Yan, Z. H. Chin. J. Org. Chem. 2015, 35, 1923 (in Chinese).(廖启华, 林森, 邓瑞红, 黄志强, 邓柯玉, 严兆华, 有机化学, 2015, 35, 1923.)
[8] (a) Hu, A. X.; Huo, S. F.; Xia, S.; Li, W.; Ye, J.; Peng, J. M.; Xiang, J. N. CN 102070556, 2011[Chem. Abstr. 2011, 154, 615156].
(b) Hu, A. X.; Li, W.; Xia, S.; Ye, J.; Huo, S. F.; Zou, S. S.; Peng, J. M.; Xiang, J. N. CN 102319244, 2012[Chem. Abstr. 2012, 156, 194844].
[9] Makam, P.; Kannan, T. Eur. J. Med. Chem. 2014, 87, 643.
[10] Turan-Zitouni, G.; Özdemir, A.; Kaplanc?kli, Z. A. Phosphorus, Sulfur, Silicon Relat. Elem. 2011, 186, 233.
[11] Kouatly, O.; Geronikaki, A.; Kamoutsis, C.; Hadjipavlou-Litina, D.; Eleftheriou, P. Eur. J. Med. Chem. 2009, 44, 1198.
[12] Siddiqui, N.; Ahsan, W. Eur. J. Med. Chem. 2010, 45, 1536.
[13] Iino, T.; Tsukahara, D.; Kamata, K.; Sasaki, K.; Ohyama, S.; Hosaka, H.; Hasegawa, T.; Chiba, M.; Nagata, Y.; Eiki, J.; Nishimura, T. Bioorg. Med. Chem. 2009, 17, 2733.
[14] Abdel-Wahab, B. F.; Mohamed, S. F.; Amr, A. E. G. E.; Abdalla, M. Monatsh. Chem. 2008, 139, 1083.
[15] Rostom, S. A. F.; Faidallah, H. M.; Radwan, M. F.; Badr, M. H. Eur. J. Med. Chem. 2014, 76, 170.
[16] Gurdal, E. E.; Durmaz, I.; Cetin-Atalay, R.; Yarim, M. J. Enzyme Inhib. Med. Chem. 2015, 30, 649.
[17] Cui, J. G.; Zhao, D. D.; He, D. M.; Huang, Y. M.; Liu, Z. P.; Lin, Q. F.; Shi, H. X.; Gan, C. F. Chin. J. Org. Chem. 2016, 36, 630 (in Chinese).(崔建国, 赵丹丹, 何冬梅, 黄燕敏, 刘志平, 林啟福, 石海信, 甘春芳, 有机化学, 2016, 36, 630.)
[18] (a) Hu, A. X.; Peng, J. M.; Fang, Y. L.; Shen, K.; Li, W.; Yan, X. W. CN 103333132, 2013[Chem. Abstr. 2013, 158, 359731].
(b) Hu, A. X.; Peng, J. M.; Shen, K.; Li, W.; Yan, X. W.; Fang, Y. L. CN 103601697, 2013[Chem. Abstr. 2013, 158, 446959].
[19] Peng, J. M. Ph.D. Dissertation, Hunan University, Changsha, 2013, (in Chinese).(彭俊梅, 博士论文, 湖南大学, 长沙, 2013.)
[20] Wu, Z. L.; Fang, Y. L.; Tang, Y. T.; Xiao, M. W.; Ye, J.; Li, G. X. A.; Hu, X. Med. Chem. Commun. 2016, 7, 1768.
[21] Peng, J. M.; Li, W.; Shen, K.; Huo, S. F.; Ye, J.; Hu, A. X. Chem. J. Chin. Univ. 2013, 34, 1646 (in Chinese).(彭俊梅, 李婉, 申坤, 霍素芳, 叶姣, 胡艾希, 高等学校化学学报, 2013, 34, 1646.)
/
| 〈 |
|
〉 |