

强碱催化分子内氮芳基化合成苯并咪唑反应机理及反应性研究
收稿日期: 2016-09-18
修回日期: 2016-12-07
网络出版日期: 2016-12-21
基金资助
国家自然科学基金(No.21202109)、四川省教育厅基金(No.13ZB0160)和四川师范大学基金(No.16ZP10)资助项目.
Theoretical Investigations on the Intramolecular N-Arylation Mechanism and Reactivity for the Synthesis of Benzimidazoles by Base-Catalyzed
Received date: 2016-09-18
Revised date: 2016-12-07
Online published: 2016-12-21
Supported by
Project supported by the National Natural Science Foundation of China (No. 21202109), the Research Fund of Department of Education, of Sichuan Province (No. 13ZB0160) and the Research Funds of Sichuan Normal University (No. 16ZP10).
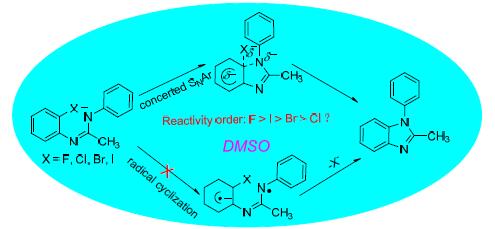
MP2/6-311+G**//B3LYP/6-311+G**理论水平上,对二甲基亚砜(DMSO)中强碱催化N-(2-卤基苯基)-N'-苯基乙脒分子内氮芳基化合成苯并咪唑反应机理及反应性做了理论研究.研究结果显示:标题反应的反应机理并不是Bolm等提出的自由基机理或分步的SNAr机理,而是只有一个过渡态的协同SNAr机理.几何结构宽松度分析和自然集居数分析(NPA)都不能解释标题反应的反应性大小顺序问题.多参数拟合揭示标题反应的反应能垒主要由最高占据轨道能EHOMO或亲核原子N10的区域亲核性指标ωN10-控制,另一因素为亲核原子N10所带负电荷多少.而被进攻的C2原子所带电荷以及几何结构宽松度L%对反应能垒几乎没有影响.方法对比研究发现,MP2/6-311+G**//B3LYP/6-311+G**方法所得结果与实验结果吻合较好,能更好地描述标题反应的相对能量和反应性顺序.
李强根 , 向仕凯 , 毛双 , 任译 . 强碱催化分子内氮芳基化合成苯并咪唑反应机理及反应性研究[J]. 有机化学, 2017 , 37(3) : 608 -616 . DOI: 10.6023/cjoc201609020
Quantum chemical studies on the intramolecular N-arylation mechanism and reactivity of N-(2-halogen phenyl)-N'-phenyl ethyl amidines in dimethyl sulfoxide (DMSO) for the synthesis of benzimidazoles by base-catalyzed have been performed at MP2/6-311+G**//B3LYP/6-311+G** level of theory. The results indicate that the mechanism of the title reactions is not the radical mechanism or stepwise SNAr pathway, but the concerted SNAr pathway with a transition state, which is compared with the conclusion of Bolm et al. The reactivity of the title reactions can not be interpreted by the geometric looseness or natural population analysis. Multi parameter fitting reveals that the reactivity of the title reactions is controlled mostly by the regional nucleophilicity index ωN10- of the nucleophile N10 atom or highest occupied molecular orbital energy EHOMO of the reactant, the other factor is the charges of the nucleophilic atom N10, while the charges of the C2 atom and the geometric looseness L% have almost no effect on the reaction energy barrier. The relative energies and the reactivity of the title reactions attained at MP2/6-311+G**//B3LYP/6-311+G** level of theory are better agreement with the experimental results compared with the other methods.

[1] Horton, D. A.; Bourne, G. T.; Smythe, M. L. Chem. Rev. 2003, 103, 893.
[2] Alamgir, M.; Black, D. St. C.; Kumar, N. Top. Heterocycl. Chem. 2007, 9, 87.
[3] Kedar, M. S.; Dighe, N. S.; Pattan, S. R.; Musmade, D. S.; Thakur, D.; Bhosale, M.; Gaware, V. M. Pharma Chem. 2010, 2, 249.
[4] Srikanth, L.; Varun Raj, V.; Raghunandan, N.; Venkateshwerlu, L. Pharma Chem. 2011, 3, 172.
[5] Narasimhan, B.; Sharma, D.; Kumar, P. Med. Chem. Res. 2012, 21, 269.
[6] Lin, S. Y.; Isome, Y.; Stewart, E.; Liu, J. F.; Yohannes, D.; Yu, L. Tetrahedron Lett. 2006, 47, 2883.
[7] Dudd, L. M.; Venardou, E.; Garcia-Verdugo, E.; Licence, P.; Blake, A. J.; Wilson, C.; Poliakoff, M. Green Chem. 2003, 5, 187.
[8] Zhang, C.; Zhang, L.; Jiao, N. Green Chem. 2012, 14, 3273.
[9] Chari, M. A.; Shobha, D.; Sasaki, T. Tetrahedron Lett. 2011, 52, 5575.
[10] Riadi, Y.; Mamouni, R.; Azzalou, R.; Haddad, M. E.; Routier, S.; Guillaumet, G.; Lazar, S. Tetrahedron Lett. 2011, 52, 3492.
[11] Chari, M. A.; Shobha, D.; Kenawy, E. R.; Al-Deyab, S. S.; Reddy, B. V. S.; Vinu, A. Tetrahedron Lett. 2010, 51, 5195.
[12] Bahrami, K.; Khodaei, M. M.; Nejatia, A. Green Chem. 2010, 12, 1237.
[13] Wan, J. P.; Gan, S. F.; Wu, J. M.; Pan, Y. Green Chem. 2009, 11, 1633.
[14] Saha, D.; Saha, A.; Ranu, B. C. Green Chem. 2009, 11, 733.
[15] Bahrami, K.; Khodaei, M. M.; Naali, F. Synlett 2009, 569.
[16] Sharghi, H.; Aberi, M.; Doroodmand, M. M. Adv. Synth. Catal. 2008, 350, 2380.
[17] Mukhopadhyay, C.; Tapaswi, P. K. Tetrahedron Lett. 2008, 49, 6237.
[18] Bahrami, K.; Khodaei, M. M.; Naali, F. J. Org. Chem. 2008, 73, 6835.
[19] Zheng, N.; Anderson, K. W.; Huang, X.; Nguyen, H. N.; Buchwald, S. L. Angew. Chem., Int. Ed. 2007, 46, 7509.
[20] Zheng, N.; Buchwald, S. L. Org. Lett. 2007, 9, 4749.
[21] Zou, B.; Yuan, Q.; Ma, D. Angew. Chem., Int. Ed. 2007, 46, 2598.
[22] Diao, X.; Wang, Y.; Jiang, Y.; Ma, D. J. Org. Chem. 2009, 74, 7974.
[23] Kim, Y.; Kumar, M. R.; Park, N.; Heo, Y., Lee, S. J. Org. Chem. 2011, 76, 9577.
[24] Evindar, G.; Batey, R. A. Org. Lett. 2003, 5, 133.
[25] Brain, C. T.; Brunton, S. A. Tetrahedron Lett. 2002, 43, 1893.
[26] Brain, C. T.; Steer, J. T. J. Org. Chem. 2003, 68, 6814.
[27] Peng, J.; Ye, M.; Zong, C.; Hu, F.; Feng, L.; Wang, X.; Wang, Y.; Chen, C. J. Org. Chem. 2011, 76, 716.
[28] Brasche, G.; Buchwald, S. L. Angew. Chem., Int. Ed. 2008, 47, 1932.
[29] Xiao, Q.; Wang, W.; Liu, G.; Meng, F.; Chen, J.; Yang, Z.; Shi, Z. Chem. Eur. J. 2009, 15, 7292.
[30] Wray, B. C.; Stambuli, J. P. Org. Lett. 2010, 12, 4576.
[31] Deng, X.; Mani, N. S. Eur. J. Org. Chem. 2010, 4, 680.
[32] Shen, M.; Driver, T. G. Org. Lett. 2008, 10, 3367.
[33] Cheng, Z.; Zhang, Q. F.; Xu, X. L.; Li, X. N. Chin. J. Org. Chem. 2015, 35(6), 1189 (in Chinese).(程正, 张群峰, 许孝良, 李小年, 有机化学, 2015, 35(6), 1189.)
[34] Zhao, D. D.; Yu, J. T.; Wang, P. C.; Lu, M. Chin. J. Org. Chem. 2016, 36(1), 165 (in Chinese). (赵丹丹, 虞家涛, 王鹏程, 陆明, 有机化学, 2016, 36(1), 165.)
[35] Yu, Z. T.; Wang, Z. Y.; Wu, X.; Hu, G. Y.; Li, Q. B. Chin. J. Org. Chem. 2016, 36(7), 1672 (in Chinese). (余祖滔, 王泽瑜, 吴肖, 胡高云, 李乾斌, 有机化学, 2016, 36(7), 1672.)
[36] Meng, Y. X.; Gui, Y. Y.; Ji, Q.; Pan, Y.; L.; Lin, Z. Q.; Lü, L.; Zeng, X. C. Chin. J. Org. Chem. 2016, 36(2), 384 (in Chinese). (蒙玉霞, 桂煜莹, 吉琼, 潘咏玲, 林志强, 吕柳, 曾向潮, 有机化学, 2016, 36(2), 384.)
[37] Yuan, Y.; Thomé, I.; Kim, S. H.; Chen, D.; Beyer, A.; Bonnamour, J.; Zuidema, E.; Chang, S.; Bolm, C. Adv. Synth. Catal. 2010, 352, 2892.
[38] Cano, R.; Ramón, D. J.; Yus, M. J. Org. Chem. 2011, 76, 654.
[39] Fang, Y.; Zheng, Y.; Wang, Z. Eur. J. Org. Chem. 2012, 7, 1495.
[40] Zou, L. H.; Reball, J.; Mottweiler, J.; Bolm, C. Chem. Commun. 2012, 48, 11307.
[41] Diness, F.; Fairlie, D. P. Angew. Chem., Int. Ed. 2012, 51, 8012.
[42] Carmen Pérez-Aguilar, M.; Valdés, C. Angew. Chem., Int. Ed. 2012, 51, 5953.
[43] Jalalian, N.; Petersen, T. B.; Olofsson, B. Chem.-Eur. J. 2012, 18, 14140.
[44] Majumdar, K. C.; Ganai, S.; Nandi, R. K.; Ray, K. Tetrahedron Lett. 2012, 53, 1553.
[45] Zhao, J.; Zhao, Y.; Fu, H. Angew. Chem., Int. Ed. 2011, 50, 3769.
[46] Beyer, A.; Reucher, C. M. M.; Bolm, C. Org. Lett. 2011, 13, 2876.
[47] Thomé, I.; Bolm, C. Org. Lett. 2012, 14, 1892.
[48] Beyer, A.; Buendia, J.; Bolm, C. Org. Lett. 2012, 14, 3948.
[49] Thom, I.; Besson, C.; Kleine, T.; Bolm, C. Angew. Chem., Int. Ed. 2013, 52, 7509.
[50] Xiang, S. K.; Tan, W.; Zhang, D. X.; Tian, X. L.; Feng, C.; Wang, B. Q.; Zhao, K. Q.; Hu, P.; Yang, H. Org. Biomol. Chem. 2013, 11, 7271.
[51] Baars, H.; Beyer, A.; Kohlhepp, S. V.; Bolm, C. Org. Lett. 2014, 16, 536.
[52] Bunnett, J. F.; Zahler, R. E. Chem. Rev. 1951, 49, 273.
[53] Terrier, F. The SNAr Reactions:Mechanistic Aspects, in Modern Nucleophilic Aromatic Substitution, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany 2013, pp. 1~84.
[54] Hunter, A.; Renfrew, M.; Taylor, J. A.; Whitmore, J. M. J.; Wil-liams, A. J. Chem. Soc., Perkin Trans. 2 1993, 1703.
[55] Fernandez, I.; Frenking, G.; Uggerud, E. J. Org. Chem. 2010, 75(9), 2971.
[56] Glukhovtsev, M. N.; Bach, R. D.; Laiter, S. J. Org. Chem. 1997, 62(12), 4036.
[57] Simkin, B. Y.; Gluz, E. B.; Glukhovtsev, M. N.; Minkin, V. I. J. Mol. Struct. (THEOCHEM) 1993, 284(1~2), 123.
[58] Becke, A. D. J. Chem. Phys. 1993, 98, 5648.
[59] Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785.
[60] Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Chem. Phys. Lett. 1989, 157, 200.
[61] Jr, J. R. P.; Veloso, D. P. Phys. Chem. Chem. Phys. 2008, 10, 1118.
[62] Cid, M. V. F.; Buijs, W.; Witkamp, G. J. Ind. Eng. Chem. Res. 2007, 46, 941.
[63] Gorelsky, S. I.; Lapointe, D.; Fagnou, K. J. Am. Chem. Sos. 2008, 130, 10848.
[64] Imoto, M.; Matsui, Y.; Takeda, M.; Tamaki, A.; Taniguchi, H.; Mizuno, K.; Ikeda, H. J. Org. Chem. 2011, 76, 6356.
[65] Toledo, R. O.; Santos, J. G.; Ríos, P.; Castro, E. A.; Campodónico, P. R.; Contreras, R. J. Phys. Chem. B 2013, 117, 5908.
[66] Toledo, R. O.; Contreras, R.; Tapiab, R. A.; Campodónico, P. R. Org. Biomol. Chem. 2013, 11, 2302.
[67] Du, L. J.; Wu, C. H.; Gu, H. H.; Li, J. J. Org. Chem. 2015, 35(8), 1726 (in Chinese). (杜丽娟, 吴彩虹, 顾红红, 李娟, 有机化学, 2015, 35(8), 1726.)
[68] Glukhovtsev, M. N.; Bach, R. D.; Laiter, S. J. Org. Chem. 1997, 62, 4036.
[69] Wadt, W. R.; Hay, P. J. J. Chem. Phys. 1985, 82, 284.
[70] Tomasi, J.; Persico, M. Chem. Rev. 1994, 94, 2027.
[71] Reed, A. E.; Curtiss, L. A.; Weinhold, F. Chem. Rev. 1988, 88, 899.
[72] Zhao, Y.; Truhlar, D. G. Theor. Chem. Acc. 2008, 120, 215.
[73] Sadowsky, D.; McNeill, K.; Cramer, C. J. Environ. Sci. Technol. 2014, 48, 10904.
[74] Cairns, A. G.; Senn, H. M.; Murphy, M. P.; Hartley, R. C. Chem. Eur. J. 2014, 20, 3742.
[75] Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. J. A; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand. J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, Ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2009.
[76] Glukhovtsev, M. N.; Pross, A.; Radom, L. J. Am. Chem. Soc. 1996, 118, 6273.
[77] Li, Q. G.; Mao, S.; Cai, W. F.; Zheng, Y.; Liu, L. X. Chemistry 2016, 79(5), 418(in Chinese). (李强根, 毛双, 蔡皖飞, 郑妍, 刘柳斜, 化学通报, 2016, 79(5), 418.)
[78] Shaik, S. S.; Schlegel, H. B.; Wolfe, S. Theoretical Aspects of Physical Organic Chemistry. The SN2 Mechanism, Wiley, New York, 1992, pp. 181~188.
[79] Reed, A. E.; Weinstock, R. B.; Weinhold, F. J. Chem. Phys. 1985, 83, 735.
[80] Chattaraj, P. K.; Sarkar, U.; Roy, D. R. Chem. Rev. 2006, 106, 2065.
[81] Ayers, P. W.; Anderson, J. S. M.; Bartolotti, L. J. Int. J. Quantum Chem. 2005, 101, 520.
[82] Contreras, R.; Andres, J.; Safont, V. S.; Campodonico, P.; Santos, J. G. J. Phys. Chem. A 2003, 107(29), 5588.
[83] Ormazábal-Toledo, R.; Contreras, R. Adv. Chem. 2014, 2014, 1.
[84] Ormazábal-Toledo, R.; Contreras, R.; Campodónico, P. R. J. Org. Chem. 2013, 78, 1091.
[85] Ormazábal-Toledo, R.; Campodónico, P. R.; Contreras, R. Org. Lett. 2011, 13, 822.
/
| 〈 |
|
〉 |