

异海松酰(胺)基硫脲类衍生物的合成及其生物活性评价
收稿日期: 2016-10-12
修回日期: 2016-12-07
网络出版日期: 2016-12-21
基金资助
国家自然科学基金(No.31370575)资助项目.
Synthesis and Bioactivity Evaluation of Acylthiourea Derivatives Based on Isopimaric Acid
Received date: 2016-10-12
Revised date: 2016-12-07
Online published: 2016-12-21
Supported by
Project supported by the National Natural Science Foundation of China (No. 31370575).
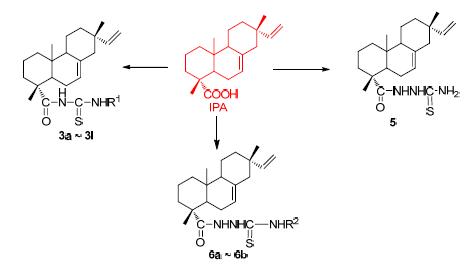
为了开发具有较高生物活性的异海松酸类衍生物,将硫脲结构拼接到异海松酸骨架上,设计合成了15个未见报道的异海松酰(胺)基硫脲类化合物,其结构经FT-IR、1H NMR、13C NMR和HRMS或元素分析确证.对化合物进行了初步的抑菌和抗癌活性测试,大多数化合物对白色念珠菌表现出显著的抑制活性,且活性高于异海松酸.在浓度为100 μmol/L时,部分化合物对人黑色素瘤(A375)和前列腺癌(PC-3)癌细胞具有明显的增殖抑制作用,尤其化合物N-异海松酰基-N'-(3-甲基苯基)硫脲(3c)和N-异海松酰胺基-N'-(4-氟苯基)硫脲(6b)对癌细胞的抑制率达到90%以上.
刘娟娟 , 卢言菊 , 王婧 , 毕良武 , 赵振东 . 异海松酰(胺)基硫脲类衍生物的合成及其生物活性评价[J]. 有机化学, 2017 , 37(3) : 731 -738 . DOI: 10.6023/cjoc201610017
To develop isopimaric acid derivatives with high bioactivity, fifteen acyl (amide) thiourea derivatives containing isopimaric acid skeleton were synthesized and confirmed by FT-IR, 1H NMR, 13C NMR and HRMS or elemental analysis. All the compounds were evaluated for their antibacterial and anticancer activity. Most of the compounds showed siginificant inhibitory activity which was higher than that of isopiamric acid against the Candida Albicans. At the concentration of 100 μmol/L, many compounds exhibited pronounced inhibitory effects on human melanoma cells (A375) and prostatic carcinoma (PC-3) cell lines, especially compounds N-isopimaric acyl-N'-(3-methylphenyl)sulfourea (3c) and N-isopimaric acylamino-N'-(4-fluorophenyl) sulfourea (6b) have the best anticancer activity, both with the inhibitory rate of over 90%.

[1] Rabergh, C. M. I.; Lilius, H.; Eriksson, J. E.; Isomaa, B. Aquat. Toxicol. 1999, 46, 55.
[2] Nikinmaa, M.; Wickström, C.; Lilius, H.; Isomaa, B.; Rabergh, C. Environ. Toxicol. Chem. 1999, 18, 993.
[3] Henney, N. C.; Li, B.; Elford, C.; Campbell, A. Am. J. Physiol. Cell Physiol. 2009, 297, C1397.
[4] Wu, C.; Gopal, K. V.; Lukas, T. J.; Gross, G. W.; Moore, E. J. Eur. J. Pharmacology 2014, 732, 68.
[5] Wang, L.; Kang, H.-C.; Li, Y.-Z.; Shui, Y.; Yamamoto, R.; Sugai, T.; Kato, N. Neuropharmacol. 2015, 92, 8.
[6] Sharma, S.; Nagar, V.; Mehta, B. K. Fitoterapia 1993, 64, 476.
[7] Tanaka, R.; Tokuda, H.; Ezaki, Y. Phytomedicine 2008, 15, 985.
[8] Chang, L. C.; Song, L. L.; Park, E. J.; Luyengi, L.; Lee, K. J.; Farnsworth, N. R.; Pezzuto, J. M.; Kinqhorn, A. D. J. Nat. Prod. 2000, 63, 1235.
[9] Adamczyk, S.; Adamczyk, B.; Kitunen, V.; Smolander, A. Soil Biol. Biochem. 2015, 87, 59.
[10] Bisio, A.; Fraternale, D.; Damonte, G.; Millo, E.; Lanteri, A. P.; Russo, E.; Romussi, G.; Parodi, B.; Ricci, D.; De Tommasi, N. Nat. Prod. Commun. 2009, 4, 1621.
[11] Elliger, C. A.; Zinkel, D. F.; Chan, B. G.; Waiss, A. C., Jr. Experientia 1976, 32, 1364.
[12] Perez Gutierrez, R. M.; Garcia, B. E. J. Asian Nat. Prod. Res. 2011, 13, 934.
[13] Cheng, S.-S.; Chang, S.-T. Wood Sci. Technol. 2014, 48, 831.
[14] Xie, Y. S.; Isman, M. B.; Yi, F.; Wong, A. J. Chem. Ecol. 1993, 19, 1075.
[15] Gong, Y.-X.; Wang, Z.-Y.; Zhang, Z.-W.; Chen, C.-B.; Wang, Y.-G. Chin. J. Org. Chem. 2006, 26, 360 (in Chinese).(龚银香, 王子云, 张正文, 陈传兵, 汪炎钢, 有机化学, 2006, 26, 360.)
[16] Zou, X.-J.; Jin, G.-Y.; Yang, Z. Chem. J. Chin. Univ. 2002, 23, 403 (in Chinese). (邹霞娟, 金桂玉, 杨昭, 高等学校化学学报, 2002, 23, 403.)
[17] Su, G.-F.; Huo, L.-N.; Qin, J.-K.; Pan, C.-X.; Guan, Y.-F. Chin. J. Appl. Chem. 2008, 25, 803 (in Chinese).(苏桂发, 霍丽妮, 覃江克, 潘成学, 关一富, 应用化学, 2008, 25, 803.)
[18] Elkholy, S. S.; Salem, H. A.; Eweis, M.; Elsabee, M. Z. Int. J. Biol. Macromol. 2014, 70, 199.
[19] Plutín, A. M.; Mocelo, R.; Alvarez, A.; Ramos, R.; Castellano, E. E.; Cominetti, M. R.; Graminha, A. E.; Ferreira, A. G.; Batista, A. A. J. Inorg. Biochem. 2014, 134, 76.
[20] Koca, ?.; Özgür, A.; Co?kun, K. A.; Tutar, Y. Bioorg. Med. Chem. 2013, 21, 3859.
[21] Wang, Y.-G.; Lu, B.-X.; Ye, W.-F.; Zhao, X.-Y.; Yang, J. Chin. J. Org. Chem. 2002, 22, 862 (in Chinese).(汪焱钢, 卢冰熙, 叶文法, 赵新筠, 杨军, 有机化学, 2002, 22, 862.)
[22] Su, G.-F.; Huo, L.-N.; Chen, R.; Zhao, F.-L.; Guan, Y.-F. Acta Chim. Sinica 2008, 66, 2717 (in Chinese).(苏桂发, 霍丽妮, 陈睿, 赵丰丽, 关一富, 化学学报, 2008, 66, 2717.)
[23] Ulubelen, A.; Oksüz, S.; Topcu, G.; Gören, A. C.; Bozok-Johansson, C.; Celik, C.; Kökdil, G.; Voelter, W. Nat. Pro. Lett. 2001, 15, 307.
[24] Zhao, Z.-D.; Li, X.-D.; Bi, L.-W.; Chen, Y.-X.; Gu, Y.; Li, D.-M.; Wang, J. J. CN 101302151, 2008[Chem. Abstr. 2008, 150, 5919].
[25] Hilliard, N. J.; Duffy, L. B.; Crabb, D. M.; Waites, K. B. J. Microbiol. Methods 2005, 60, 285.
[26] Liu, Z.-J.; Wu, S.-S.; Wang, Y.; Li, R.-J.; Wang, J.; Wang, L.-H.; Zhao, Y.-F.; Gong, P. Eur. J. Med. Chem. 2014, 87, 782.
/
| 〈 |
|
〉 |