

含亚胺基(E)-β-Farnesene类似物的设计、合成及生物活性研究
收稿日期: 2016-11-22
修回日期: 2016-12-18
网络出版日期: 2016-12-21
基金资助
国家自然科学基金重点(No.21132003)资助项目.
Design, Synthesis and Biological Activity of (E)-β-Farnesene Analogues Containing Imidogen
Received date: 2016-11-22
Revised date: 2016-12-18
Online published: 2016-12-21
Supported by
Project supported by the National Natural Science Foundation of China (No. 21132003).
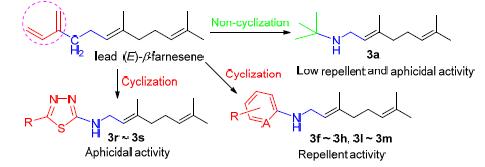
为了发现控制蚜虫的新型高活性化合物,以蚜虫报警信息素(E)-β-farnesene (EBF)为先导,引入不同类型的芳香环(叔丁基)替代EBF结构中不稳定的共轭双键,设计了一系列含亚胺基EBF类似物. 采用N-烷基化反应和还原胺化反应两种路线合成了19个目标化合物. 所有化合物结构均通过1HNMR、13CNMR、IR及HRMS确证. 对目标物进行了生物活性测试并探讨了结构活性关系,结果表明:含苯环或吡啶环的化合物对桃蚜具有较好的驱避活性,其中以3g、3l和3m最为显著,驱避率分别为62.0%、62.5%和64.6%;含1,3,4-噻二唑等杂环的化合物对桃蚜具有一定的杀虫活性,在200 μg/mL浓度下化合物3i、3l、3r和3s的致死率分别为70.7%、72.6%、70.4%和75.5%,显著高于先导EBF;而含叔丁基的非环EBF类似物的驱避和杀蚜活性均不明显.
张景朋 , 秦耀果 , 李欣潞 , 宋敦伦 , 刘俊杰 , 杨新玲 . 含亚胺基(E)-β-Farnesene类似物的设计、合成及生物活性研究[J]. 有机化学, 2017 , 37(4) : 987 -995 . DOI: 10.6023/cjoc201611028
In order to discover novel compounds with high-activity to control aphid, aphid alarm pheromone (E)-β-farnesene (EBF) was chosen as lead compound and a series of EBF analogues containing imidogen were designed by replacing unstable conjugated double bonds of EBF with different aromatic ring. Nineteen EBF analogues were synthesized via two reaction routes of N-alkylation and reductive amination. Their structures were confirmed by 1H NMR, 13C NMR, IR and HRMS analysis. The bioassay and structure-activity relationship analysis were carried out. The result indicated that title compounds containing benzene or pyridine displayed obvious repellent activity against Myzus persicae (Sulzer). Especially, compounds 3g, 3l and 3m exhibited excellent repellent activity of 62.0%, 62.5% and 64.6% respectively. Moreover, the title compounds containing 1,3,4-thiadiazole ring showed certain insecticidal activity against M. persicae. In particular, the mortality rate of compounds 3i, 3l, 3r and 3s were 70.7%, 72.6%, 70.4% and 75.5% at a concentration of 200 μg/mL, which were higher than the lead EBF. However, compound 3a, in which the conjugated double bonds were replaced with butyl, showed obviously neither repellent nor aphicidal activity.

[1] Tang, P.-H.; Chen, G.-P.; Zhu, M.-K.; Ren, L.-J.; Hu, Z.-L. J. Plant Prot. 2013, 39, 5 (in Chinese).
(唐平华, 陈国平, 朱明库, 任丽军, 胡宗利, 植物保护, 2013, 39, 5.)
[2] Wang, G.-P.; Yu, X.-D.; Fan, J.; Wang, C.-S.; Xia, L.-Q. J. Integr. Plant Biol. 2015, 57, 770.
[3] Dahl, M. L. Dtsch. Ent. Z. 1971, 18, 121.
[4] Bowers, W. S.; Nault, L. R.; Webb, R. E.; Dutky, S. R. Science 1972, 177, 1121.
[5] Edwards, L.; Siddall, J.; Dunham, L. Nature 1973, 241, 126.
[6] Mauchamp, B.; Pickett, J. J. Agronomie 1987, 7, 523.
[7] Van Oosten, A. M.; Gut, J.; Harrewijn, P.; Piron, P. G. M. Acta Phytopathol. Entomol. Hung. 1990, 25, 331.
[8] Joachim, C.; Weisser, W. W. J. Chem. Ecol. 2015, 47, 267.
[9] Joachim, C.; Hatano, E.; David, A.; Kunert, M.; Linse, C.; Weisser, W. W. J. Chem. Ecol. 2013, 39, 773.
[10] Griffiths, D. C.; Pickett, J. A. Entomol. Exp. Appl. 1980, 27, 199.
[11] Dawson, G. W.; Giffiths, D. C.; Pickett, J. A.; Plumb, R. T.; Woodcock, C. M.; Zhang, Z.-N. Pestic. Sci.. 1988, 22, l7.
[12] Bowers, W. S.; Nishino, C.; Montgomery, M. E.; Nault, L. R. Insect Physiol. 1977, 23, 697.
[13] Kang, T.-N.; Ling, Y.; Rui, C.-H.; Yang, X.-L.; Fan, X.-L.; Chen, F.-H. Chin. J. Org. Chem. 2008, 28, 617 (in Chinese).
(康铁牛, 凌云, 芮昌辉, 杨新玲, 范贤林, 陈馥衡, 有机化学, 2008, 28, 617.)
[14] Li, Z.-M.; Wang, T.-S.; Me, E.-Y.; Chen, X.-R.; Zhu, L.-H.; Wang, S.-H. Acta Chim. Sinica 1987, 45, 1124 (in Chinese).
(李正名, 王天生, 么恩云, 陈学仁, 朱兰蕙, 王素华, 化学学报, 1987, 45, 1124.)
[15] Sun, L.; Ling, Y.; Wang. C.; Sun, Y.-F.; Rui, C.-H.; Yang, X.-L. Chin. J. Org. Chem. 2011, 31, 2061 (in Chinese).
(孙亮, 凌云, 王灿, 孙玉凤, 芮昌辉, 杨新玲, 有机化学, 2011, 31, 2061.)
[16] Sun, Y.-F.; Li, Y.-Q.; Ling, Y.; Yu, H.-L.; Yang, S.-X.; Yang, X.-L. Chin. J. Org. Chem. 2011, 31, 1425 (in Chinese).
(孙玉凤, 李永强, 凌云, 宇红莲, 杨绍祥, 杨新玲, 有机化学, 2011, 31, 1425.)
[17] Sun, Y.-F.; Qiao, H.-L.; Ling, Y.; Yang, S.-X.; Rui, C.-H.; Paolo Pelosi; Yang, X.-L. J. Agric. Food Chem. 2011, 59, 2456.
[18] Qin, Y.-G.; Qu, Y.-Y.; Zhang, J.-P.; Tan, X.-Q.; Song, L.-F.; Li, W.-H.; Song, D.-L.; Yang, X.-L. Chin. J. Org. Chem. 2015, 35, 455 (in Chinese).
(秦耀果, 曲焱焱, 张景朋, 谭晓庆, 宋丽芳, 李文浩, 宋敦伦, 杨新玲, 有机化学, 2015, 35, 455.)
[19] Qin, Y.-G.; Zhang, J.-P.; Yang, X. L. Molecules 2016, 21, 825.
[20] Zhang, J.-P.; Qin, Y.-G.; Li, W.-H.; Ling, Y.; Yang, L.-B.; Song, D.-L.; Yang, X.-L. Chin. J. Org. Chem. 2016, 36, 1883 (in Chinese).
(张景朋, 秦耀果, 李文浩, 杨立波, 宋敦伦, 杨新玲, 有机化学, 2016, 36, 1883.)
[21] Peat, A. J.; Buchwald, S. L. J. Am. Chem. Soc. 1996, 118, 1028.
[22] Romera, J. L.; Cid, J. M.; Trabanco, A. A. Tetrahedron Lett. 2004, 45, 8797.
[23] Cunha, C. R. M. D.; Neto, S. A. M.; Silva, C. C. D.; Cortez, A. P.; Gomes, M. D. N.; Martins, F. I.; Alonso, A.; Rezende, K. R.; Menegatti, R.; Magalhaes, M. T. Q. D. M.; Valadares, M. C. Eur. J. Med. Chem. 2013, 62, 371.
[24] Masatoshi, H. J. Chem. Ecol. 1988, 24, 1425.
[25] Zhang, C.-L.; Qu, Y.-Y.; Wu, X.-Q.; Song, D.-L.; Ling, Y.; Yang, X. L. J. Agric. Food Chem. 2015, 63, 4527.
/
| 〈 |
|
〉 |