

吡啶查尔酮衍生物的合成及抑制耐甲氧西林金黄色葡萄球菌活性评价
收稿日期: 2016-10-11
修回日期: 2016-12-21
网络出版日期: 2016-12-29
基金资助
国家自然科学基金(Nos.U1204206,81501782)和河南省教育厅(No.17A350004)资助项目.
Synthesis and Antibiotic Activity Study of Pyridine Chalcone Derivatives against Methicillin-Resistant Staphylococcus aureus
Received date: 2016-10-11
Revised date: 2016-12-21
Online published: 2016-12-29
Supported by
Project supported by the National Natural Science Foundation of China (Nos. U1204206, 81501782) and the Education Department of Henan Province (No. 17A350004).
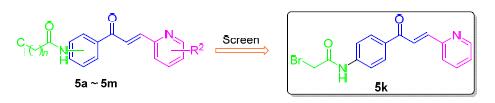
合成了一系列13个吡啶查尔酮衍生物,目标化合物的结构通过1H NMR、13C NMR和 HRMS进行了确证. 体外抗菌活性评价结果显示,其中的五个化合物对于革兰氏阳性金黄色葡萄球菌(ATCC 29213)表现出良好的抑制活性. 为进一步评估活性,选取11株无重复的耐甲氧西林金黄色葡萄球菌(MRSA)临床株对上述5个化合物进行进一步的药效测评. 数据显示,其中四个化合物对于MRSA表现出较好的抑菌活性,其中(E)-2-溴-N-{4-[3-(2-吡啶基)丙烯酰基]苯基}乙酰胺(5k)对MRSA的抑菌效力最为明显,其最小抑菌浓度(MIC)为4 μg/mL. 在安全性评价方面,对化合物5k进行红细胞毒性评价,结果显示化合物5k即使在1000 μg/mL的浓度下对于红细胞也几乎不存在毒性. 综合上述数据分析,吡啶查尔酮5k作为新型抗MRSA药物具有进一步研究的价值.
关键词: 吡啶查尔酮; 耐甲氧西林金黄色葡萄球菌; 抗菌活性; 红细胞溶血
张恩 , 王铭铭 , 徐帅民 , 王上 , 赵娣 , 白鹏燕 , 崔得运 , 化永刚 , 王亚娜 , 秦上尚 , 刘宏民 . 吡啶查尔酮衍生物的合成及抑制耐甲氧西林金黄色葡萄球菌活性评价[J]. 有机化学, 2017 , 37(4) : 959 -966 . DOI: 10.6023/cjoc201610016
A series of pyridine chalcone derivatives were designed and synthesized. The structures were confirmed by 1H NMR, 13C NMR and HRMS. In vitro biological activity evaluation results showed that five of the compounds exhibited good biological activity against gram positive bacteria staphylococcus aureus (ATCC 29213). Further antibiotic activity of these five compounds against 11 clinical isolated methicillin-resistant staphylococcus aureus (MRSA) were evaluated. The results showed that four of the compounds exhibited good antibacterial activity against MRSA. (E)-2-Bromo-N-{4-[3-(pyridin-2-yl)- acryloyl]phenyl}acetamide (5k) showed the most effective activity (minimum inhibitory concentration, MIC=4 μg/mL). In terms of hemolytic activity evaluation, compound 5k showed virtually no toxicity even in 1000 μg/mL concentration. To sum up, pyridine chalcone 5k was potential for further antibiotic study as anti-MRSA agent.

[1] Jevons, M. P. Br. Med. J. 1961, 1, 124.
[2] Hamilton, G. L. A. Lab. Med. 2010, 41, 329.
[3] Deurenberg, R. H.; Nulens, E.; Valvatne, H.; Sebastian, S.; Driessen, C.; Craeghs, J.; De Brauwer, E.; Heising, B.; Kraat, Y. J.; Riebe, J.; Stals, F. S.; Trienekens, T. A.; Scheres, J.; Friedrich, A. W.; van Tiel, F. H.; Beisser, P. S.; Stobberingh, E. E. Emerging Infect. Dis. 2009, 15, 727.
[4] Kallen, A. J.; Mu, Y.; Bulens, S.; Reingold, A.; Petit, S.; Gershman, K.; Ray, S. M.; Harrison, L. H.; Lynfield, R.; Dumyati, G. JAMA, J. Am. Med. Assoc. 2010, 304, 641.
[5] Fridkin, S. K.; Hageman, J. C.; Morrison, M.; Sanza, L. T.; Como-Sabetti, K.; Jernigan, J. A.; Harriman, K.; Harrison, L. H.; Lynfield, R.; Farley, M. M. N. Engl. J. Med. 2005, 352, 1436.
[6] Cardo, D.; Horan, T.; Andrus, M.; Dembinski, M.; Edwards, J.; Peavy, G.; Tolson, J.; Wagner, D. Am. J. Infect. Control. 2004, 32, 470.
[7] Fridkin, S. K.; Yokoe, D. S.; Whitney, C. G.; Onderdonk, A.; Hooper, D. C. J. Clin. Microbiol. 1998, 36, 965.
[8] Rybak, M. J.; Abate, B. J.; Kang, S. L.; Ruffing, M. J.; Lerner, S. A.; Drusano, G. L. Antimicrob. Agents Chemother. 1999, 43, 1549.
[9] Nowakowska, Z. Eur. J. Med. Chem. 2007, 42, 125.
[10] Magiorakos, A. P.; Srinivasan, A.; Carey, R. B.; Carmeli, Y.; Falagas, M. E.; Giske, C. G.; Harbarth, S.; Hindler, J. F.; Kahlmeter, G.; Olsson-Liljequist, B.; Paterson, D. L.; Rice, L. B.; Stelling, J.; Struelens, M. J.; Vatopoulos, A.; Weber, J. T.; Monnet, D. L. Clin. Microbiol. Infect. 2012, 18, 268.
[11] Kluytmans, J.; Van Belkum, A.; Verbrugh, H. Clin. Microbiol. Rev. 1997, 10, 505.
[12] Patel, R. V.; Patel, P. K.; Kumari, P.; Rajani, D. P.; Chikhalia, K. H. Eur. J. Med. Chem. 2012, 53, 41.
[13] Chen, Z.-H.; Zheng, C.-J.; Sun, L.-P.; Piao, H.-R. Eur. J. Med. Chem. 2010, 45, 5739.
[14] Klevens, R. M.; Morrison, M. A.; Nadle, J.; Petit, S.; Gershman, K.; Ray, S.; Harrison, L. H.; Lynfield, R.; Dumyati, G.; Townes, J. M. JAMA, J. Am. Med. Assoc. 2007, 298, 1763.
[15] Zhang, Q.-R.; Xue, D.-Q.; He, P.; Shao, K.-P.; Chen, P.-J.; Gu, Y.-F.; Ren, J.-L.; Shan, L.-H.; Liu, H.-M. Bioorg. Med. Chem. Lett. 2014, 24, 1236.
[16] Chen, P.-J.; Yang, A.; Gu, Y.-F.; Zhang, X.-S.; Shao, K.-P.; Xue, D.-Q.; He, P.; Jiang, T.-F.; Zhang, Q.-R.; Liu, H.-M. Bioorg. Med. Chem. Lett. 2014, 24, 2741.
[17] Nielsen, S. F.; Larsen, M.; Boesen, T.; Schønning, K.; Kromann, H. J. Med. Chem. 2005, 48, 2667.
[18] Stringer, J. R.; Bowman, M. D.; Weisblum, B.; Blackwell, H. E. ACS Comb. Sci. 2011, 13, 175.
[19] Joshi, A. S.; Li, X. C.; Nimrod, A. C.; ElSohly, H. N.; Walker, L. A.; Clark, A. M. Planta Med. 2001, 67, 186.
[20] Fu, D.-J.; Zhang, S.-Y.; Liu, Y.-C.; Yue, X.-X.; Liu, J.-J.; Song, J.; Zhao, R.-H.; Li, F.; Sun, H.-H.; Zhang, Y.-B.; Liu, H.-M. Med. Chem. Commun. 2016, 7, 1664.
[21] Fu, D.-J.; Zhang, S.-Y.; Liu, Y.-C.; Zhang, L.; Liu, J.-J.; Song, J.; Zhao, R.-H.; Li, F.; Sun, H.-H.; Liu, H.-M.; Zhang, Y.-B. Bioorg. Med. Chem. Lett. 2016, 26, 3918.
[22] Ling, L. L.; Schneider, T.; Peoples, A. J.; Spoering, A. L.; Engels, I.; Conlon, B. P.; Mueller, A.; Schaberle, T. F.; Hughes, D. E.; Epstein, S.; Jones, M.; Lazarides, L.; Steadman, V. A.; Cohen, D. R.; Felix, C. R.; Fetterman, K. A.; Millett, W. P.; Nitti, A. G.; Zullo, A. M.; Chen, C.; Lewis, K. Nature 2015, 517, 455.
[23] Ghosh, C.; Manjunath, G. B.; Akkapeddi, P.; Yarlagadda, V.; Hoque, J.; Uppu, D. S.; Konai, M. M.; Haldar, J. J. Med. Chem. 2014, 57, 1428.
/
| 〈 |
|
〉 |