

3-异硫氰酸酯氧化吲哚参与的不对称串联反应研究进展
收稿日期: 2016-11-15
修回日期: 2016-12-21
网络出版日期: 2017-01-04
基金资助
国家自然科学基金(No.21602052)、湖北省教育厅科学技术研究(No.Q20163004)资助项目.
Recent Advances in 3-Isothiocyanato Oxindoles Engaged Asymmetric Cascade Reactions
Received date: 2016-11-15
Revised date: 2016-12-21
Online published: 2017-01-04
Supported by
Project supported by the National Natural Science Foundation of China (No. 21602052) and the Scientific Research Project of Hubei Provincial Department of Education (No. Q20163004).
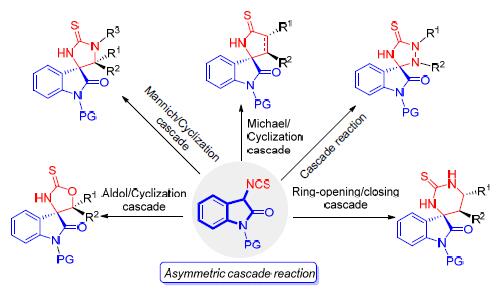
3-异硫氰酸酯氧化吲哚是一类高活性的新型反应试剂,已经被广泛地应用于串联反应中,并用于结构多样的手性螺环氧化吲哚骨架的构建. 简单综述了近六年来3-异硫氰酸酯氧化吲哚参与的几类串联环化反应的最新研究进展,主要介绍各反应的特点、活化模式及合成应用,并展望它的发展前景.
关键词: 3-异硫氰酸酯氧化吲哚; 螺环氧化吲哚; 不对称合成; 串联反应
谭芬 , 肖文精 , 曾国平 . 3-异硫氰酸酯氧化吲哚参与的不对称串联反应研究进展[J]. 有机化学, 2017 , 37(4) : 824 -840 . DOI: 10.6023/cjoc201611017
3-Isothiocyanato oxindoles have been widely employed as a class of highly reactive and novel reagents in the enantioselective synthesis of diverse spirooxindoles. This review summarizes the recent advances of 3-isothiocyanato oxindoles mediated some types of cascade process in the past six years, including properties of reaction, activation models and synthetic applications. Furthermore, the prospects of this concept are also discussed.

[1] Lin, H.; Danishefsky, S. J. Angew. Chem., Int. Ed. 2003, 42, 36.
[2] Marti, C.; Carreira, E. M. Eur. J. Org. Chem. 2003, 2209.
[3] (a) Zhou, F.; Liu, Y.-L.; Zhou, J. Adv. Synth. Catal. 2010, 352, 1381.
(b) Yu, J.; Shi, F.; Gong, L.-Z. Acc. Chem. Res. 2011, 44, 1156.
(c) Rios, R. Chem. Soc. Rev. 2012, 41, 1060.
(d) Cheng, D.-J.; Ishihara, Y.; Tan, B.; Barbas III, C. F. ACS Catal. 2014, 4, 743.
(e) Xiao, Y.-L.; Zhou, Y.; Wang, J.; Wang, J.-X.; Liu, H. Chin. J. Org. Chem. 2015, 35, 2035 (in Chinese).
(肖永龙, 周宇, 王江, 王进欣, 柳红, 有机化学, 2015, 35, 2035.)
[4] Suchy, M.; Kutschy, P.; Monde, K. J. Org. Chem. 2001, 66, 3940.
[5] (a) Cui, C. B.; Kakeya, H.; Osada, H. J. Antibiot. 1996, 49, 832.
(b) Edmondson, S.; Danishefsky, S.-J.; Sepp-Lorenzino, L.; Rosen, N. J. Am. Chem. Soc. 1999, 121, 2147.
(c) Ding, K.; Lu, Y.; Nikolovska-Coleska, Z.; Qiu, S.; Ding, Y.; Gao, W.; Stuckey, J.; Krajewski, K.; Roller, P. P.; Tomita, Y.; Parrish, D. A.; Deschamps, J. R.; Wang, S. J. Am. Chem. Soc. 2005, 127, 10130.
(d) Cheng, M.-N.; Wang, H.; Gong, L.-Z. Org. Lett. 2011, 13, 2418.
[6] Jossang, A.; Jossang, P.; Hadi, H. A.; Sevenet, T.; Bodo, B. J. Org. Chem. 1991, 56, 6527.
[7] (a) Potawel, S. E.; Mehta, U. K.; Waseem, S.; Dhalawat, H. J.; Lunya, K. P.; Mantri, R. A.; Vetol, Y. D. Pharmacology 2008, 2, 197.
(b) Litvinov, Y. M.; Mortikov, V. Y.; Shestopalov, A. M. J. Comb. Chem. 2008, 10, 741.
[8] Rottmann, M.; McNamara, C.; Yeung, B. K. S.; Lee, M. C. S.; Zou, B.; Russell, B.; Seitz, P.; Plouffe, D. M.; Dharia, N. V.; Tan, J.; Cohen, S. B.; Spencer, K. R.; González-Páez, G. E.; Lakshminarayana, S. B.; Goh, A.; Suwanarusk, R.; Jegla, T.; Schmitt, E. K.; Beck, H. P.; Brun, R.; Nosten, F.; Renia, L.; Dartois, V.; Keller, T. H.; Fidock, D. A.; Winzeler, E. A.; Diagana, T. T. Science 2010, 329, 1175.
[9] Liang, H.; Li, R.-M.; Yuan, Q.-P. J. Beijing Univ. Chem. Technol. (Nat. Sci.) 2015, 42, 1 (in Chinese).
(梁浩, 李瑞敏, 袁其朋, 北京化工大学学报(自然科学版), 2015, 42, 1.)
[10] (a) Chen, W.-B.; Wu, Z.-J.; Hu, J.; Cun, L.-F.; Zhang, X.-M.; Yuan, W.-C. Org. Lett. 2011, 13, 2472.
(b) Han, W.-Y.; Zhao, J.-Q.; Zuo, J.; Xu, X.-Y.; Zhang, X.-M.; Yuan, W.-C. Adv. Synth. Catal. 2015, 357, 3007.
[11] Jiang, K.; Jia, Z.-J.; Yin, X.; Wu, L.; Chen, Y.-C. Org. Lett. 2010, 12, 2766.
[12] Han, Y.-Y.; Chen, W.-B.; Han, W.-Y.; Wu, Z.-J.; Zhang, X.-M.; Yuan, W.-C. Org. Lett. 2012, 14, 490.
[13] Kayal, S.; Mukherjee, S. Org. Lett. 2015, 17, 5508.
[14] Chen, W.-B.; Han, W.-Y.; Han, Y.-Y.; Zhang, X.-M.; Yuan, W.-C. Tetrahedron 2013, 69, 5281.
[15] (a) Kato, S.; Kanai, M.; Matsunaga, S. Chem. Asian J. 2013, 8, 1768.
(b) Kato, S.; Kanai, M.; Matsunaga, S. Heterocycles 2014, 88, 475.
[16] Kato, S.; Yoshino, T.; Shibasaki, M.; Kanai, M.; Matsunaga, S. Angew. Chem., Int. Ed. 2012, 51, 7007.
[17] (a) Vassilev, L. T.; Vu, B. T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C.; Fotouhi, N.; Liu, E. A. Science 2004, 303, 844.
(b) Tovar, C.; Rosinski, J.; Filipovic, Z.; Higgins, B.; Kolinsky, K.; Hilton, H.; Zhao, X.; Vu, B. T.; Qing, W.; Packman, K.; Myklebost, O.; Heimbrook, D. C.; Vassilev, L. T. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 1888.
[18] Shangary, S.; Qin, D.; McEachern, D.; Liu, M.; Miller, R. S.; Qiu, S.; Nikolovska-Coleska, Z.; Ding, K.; Wang, G.; Chen, J.; Bernard, D.; Zhang, J.; Lu, Y.; Gu, Q.; Shah, R. B.; Pienta, K. J.; Ling, X.; Kang, S.; Guo, M.; Sun, Y.; Yang, D.; Wang, S. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 3933.
[19] Cai, H.; Zhou, Y.; Zhang, D.; Xu, J.-Y.; Liu, H. Chem. Commun. 2014, 50, 14771.
[20] Bai, M.; Cui, B.-D.; Zuo, J.; Zhao, J.-Q.; You, Y.; Chen, Y.-Z.; Xu, X.-Y.; Zhang, X.-M.; Yuan, W.-C. Tetrahedron 2015, 71, 949.
[21] Du, D.; Xu, Q.; Li, X.-G.; Shi, M. Chem. Eur. J. 2016, 22, 4733.
[22] Cao, Y.-M.; Shen, F.-F.; Zhang, F.-T.; Wang, R. Chem. Eur. J. 2013, 19, 1184.
[23] Wu, H.; Zhang, L.-L.; Tian, Z.-Q.; Huang, Y.-D.; Wang, Y.-M. Chem. Eur. J. 2013, 19, 1747.
[24] Tan, F.; Cheng, H.-G.; Feng, B.; Zou, Y.-Q.; Duan, S.-W.; Chen, J.-R.; Xiao, W.-J. Eur. J. Org. Chem. 2013, 2071.
[25] Wu, S.; Zhu, X.-L.; He, W.-J.; Wang, R.-M.; Xie, X.-H.; Qin, D.-B.; Jing, L.-H.; Chen, Z.-Q. Tetrahedron 2013, 69, 11084.
[26] Tan, F.; Lu, L.-Q.; Yang, Q.-Q.; Guo, W.; Bian, Q.; Chen, J.-R.; Xiao, W.-J. Chem. Eur. J. 2014, 20, 3415.
[27] Fu, Z.-K.; Pan, J.-Y.; Xu, D.-C.; Xie, J.-W. RSC Adv. 2014, 4, 51548.
[28] Kayal, S.; Mukherjee, S. Eur. J. Org. Chem. 2014, 6696.
[29] (a) Zhao, J.-Q.; Zhou, M.-Q.; Wu, Z.-J.; Wang, Z.-H.; Yue, D.-F.; Xu, X.-Y.; Zhang, X.-M.; Yuan, W.-C. Org. Lett. 2015, 17, 2238.
(b) Zhao, J.-Q.; Wu, Z.-J.; Zhou, M.-Q.; Xu, X.-Y.; Zhang, X.-M.; Yuan, W.-C. Org. Lett. 2015, 17, 5020.
[30] Du, D.; Jiang, Y.; Xu, Q.; Shi, M. Adv. Synth. Catal. 2013, 355, 2249.
[31] Wang, L.-Q.; Yang, D.-X.; Li, D.; Liu, X.-H.; Zhao, Q.; Zhu, R.-R.; Zhang, B.-Z.; Wang, R. Org. Lett. 2015, 17, 4260.
[32] Du, D.; Jiang, Y.; Xu, Q.; Tang, X.-Y.; Shi, M. ChemCatChem 2015, 7, 1366.
[33] Chowdhury, R.; Kumar, M.; Ghosh, S. K. Org. Biomol. Chem. 2016, 14, 11250.
[34] Liu, X.-L.; Han, W.-Y.; Zhang, X.-M.; Yuan, W.-C. Org. Lett. 2013, 15, 1246.
[35] Han, W.-Y.; Li, S.-W.; Wu, Z.-J.; Zhang, X.-M.; Yuan, W.-C. Chem. Eur. J. 2013, 19, 5551.
[36] Chen, Q.; Liang, J.-Y.; Wang, S.-L.; Wang, D.; Wang, R. Chem. Commun. 2013, 49, 1657.
[37] Cui, B.-D.; Li, S.-W.; Zuo, J.; Wu, Z.-J.; Zhang, X.-M.; Yuan, W.-C. Tetrahedron 2014, 70, 1895.
[38] Zhao, H.-W.; Tian, T.; Pang, H.-L.; Li, B.; Chen, X.-Q.; Yang, Z.; Meng, W.; Song, X.-Q.; Zhao, Y.-D.; Liu, Y.-Y. Adv. Synth. Catal. 2016, 358, 2619.
[39] Kayal, S.; Mukherjee, S. Org. Biomol. Chem. 2016, 14, 10175.
[40] Liu, L.; Zhao, B.-L.; Du, D.-M. Eur. J. Org. Chem. 2016, 4711.
[41] Jiang, Y.; Pei, C.-K.; Du, D.; Li, X.-G.; He, Y.-N.; Xu, Q.; Shi, M. Eur. J. Org. Chem. 2013, 7895.
[42] Wang, L.-Q.; Yang, D.-X.; Li, D.; Wang, R. Org. Lett. 2015, 17, 3004.
[43] Zhu, G.-M.; Sun, W.-S.; Wu, C.-Y.; Li, G.-F.; Hong, L.; Wang, R. Org. Lett. 2013, 15, 4988.
/
| 〈 |
|
〉 |