

CuI催化无溶剂一锅法合成苯二氮?酮类化合物
收稿日期: 2016-11-18
修回日期: 2016-12-14
网络出版日期: 2017-01-04
One Pot Solvent-Free Synthesis of Benzodiazepinones Catalyzed by CuI
Received date: 2016-11-18
Revised date: 2016-12-14
Online published: 2017-01-04
张青扬 , 汪小涧 , 肖琼 , 尹大力 . CuI催化无溶剂一锅法合成苯二氮?酮类化合物[J]. 有机化学, 2017 , 37(4) : 954 -958 . DOI: 10.6023/cjoc201611020
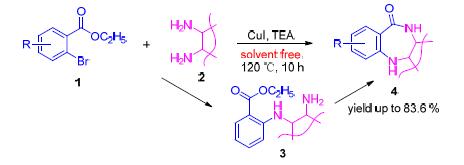
Benzodiazepinones are the important class of organic heterocyclic compounds with physiological activities. Herein, one pot procedure for the synthesis of benzodiazepinones has been developed under solvent-free condition. The substituted ethyl 2-bromobenzoates were cross-coupled with adjacent diamine compounds using CuI as a catalyst to give the intermediates, which spontaneously underwent an intramolecular N-acylation producing corresponding benzodiazepinones. This method has the advantages of enviroment frendly, mild reaction conditions, simple one pot operation and high yields. Under the optimized conditions, the effect of various substituted group on the reaction was investigated and the tolerance of this system was evaluated. This protocol could tolerate a variety of functional groups, and provide efficient access to a wide variety of substituted benzodiazepinones in good yields, including biological active molecules.
Key words: solvent-free; one pot; CuI; benzodiazepinones; catalyzed-coupling reaction
[1] (a) Shen, S.-C.; Sun, X.-W.; Lin, G.-Q. Green Chem. 2013, 15, 896.
(b) DeSimone, J. M. Science 2002, 297, 799.
(c) Jeon, S. J.; Li, H.; Walsh, P. J. J. Am. Chem. Soc. 2005, 127, 16416.
[2] (a) Poliakoff, M.; Anastas, P. Nature 2001, 413, 257.
(b) Gross, R. A.; Kalra, B. Science 2002, 297, 803.
(c) Wang, D.; Li, L.; Li, N.; Gao, T. T.; Hou, S. H.; Chen, B. H. Green Chem. 2010, 12, 45.
[3] (a) Singh, M. S.; Nagaraju, A.; Verma, G. K.; Shukla, G.; Verma, R. K.; Srivastava, A.; Raghuvanshi, K. Green Chem. 2013, 15, 954.
(b) Horváth, I. T. Green Chem. 2008, 10, 1024.
[4] (a) Sanchez, Y.; Wong, C.; Thoma, R. S.; Richman, R.; Wu, Z.; Piwnica-Worms, H.; Elledge, S. J. Science 1997, 277, 1497.
(b) Xiao, Z.; Chen, Z.; Gunasekera, A. H.; Sowin, T. J.; Rosenberg, S. H.; Fesik, S.; Zhang, H. J. Biol. Chem. 2003, 278, 21767.
[5] Hussenether, T.; Hübner, H.; Gmeiner, P.; Troschütz, R. Bioorg. Med. Chem. 2004, 12, 2625.
[6] Misiti, D.; Gatta, F.; Landi-Vittory, R. J. Heterocycl. Chem. 1971, 8, 231.
[7] (a) Cortés, E. C.; Islas, P. M.; Romero, M. O. Z. J. Heterocycl. Chem. 1996, 33, 1723.
(b) Bunce, R. A.; Schammerhorn, J. E. J. Heterocycl. Chem. 2006, 37, 1031.
[8] Wang, L.; Sullivan, G. M.; Hexamer, L. A.; Hasvold, L. A.; Thalji, R.; Przytulinska, M.; Tao, Z. F.; Li, G.; Chen, Z.; Xiao, Z.; Gu, W. Z.; Xue, J.; Bui, M. H.; Merta, P.; Kovar, P.; Bouska, J. J.; Zhang, H.; Park, C.; Stewart, K. D.; Sham, H. L.; Sowin, T. J.; Rosenberg, S. H.; Lin, N. H. J. Med. Chem. 2007, 50, 4162.
[9] (a) Liu, Y.-Y.; Wan, J.-P. Org. Biomol. Chem. 2011, 9, 6873.
(b) Liu, Y.-Y.; Wan, J.-P. Chem. Asian J. 2012, 7, 1488.
[10] Klapars, A.; Parris, S.; Anderson, K. W.; Buchwald, S. L. J. Am. Chem. Soc. 2004, 126, 3529.
[11] Xie, X.; Chen, Y.; Ma, D. J. Am. Chem. Soc. 2006, 128, 16050.
[12] Zhang, Q. Y.; Wang, X.-J.; Tian, Y.-L.; Qi, J.-G.; Li, C.; Yin, D.-L. Chin. Chem. Lett. 2013, 24, 825.
[13] Binaschi, M.; Boldetti, A.; Gianni, M.; Maggi, C. A.; Gensini, M.; Bigioni, M.; Parlani, M.; Giolitti, A.; Fratelli, M.; Valli, C.; Mineko, T.; Garattini, E. ACS Med. Chem. Lett. 2010, 1, 411.
/
| 〈 |
|
〉 |