

桥联双萘及其衍生物的合成以及对有机阳离子的络合作用
收稿日期: 2016-12-08
修回日期: 2016-12-27
网络出版日期: 2017-01-04
基金资助
国家自然科学基金(Nos.21302090,21572097)和中组部“青年千人计划”资助项目.
Synthesis of Bis-naphthalene and Their Derivatives and Their Complexation with Organic Cation
Received date: 2016-12-08
Revised date: 2016-12-27
Online published: 2017-01-04
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21302090, 21572097) and the Thousand Talents Program-Youth.
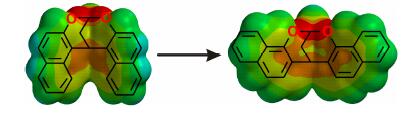
对基于2-萘酚和丙二缩醛的桥联双萘进行了改造,合成了六种具有不同取代基、侧壁或桥的桥联双萘衍生物.通过单晶衍射和分子模拟对其结构进行了研究,所有化合物都具有弧形结构.静电势能表面表明这些弧形分子的内部是富电子的,因此能够通过阳离子-π相互作用与有机阳离子发生络合.通过1H NMR滴定和Job's曲线研究了这些弧形分子与基于1,4-二氮杂二环[2.2.2]辛烷(DABCO)的有机阳离子的键合计量比和络合常数.结果表明富电子的分子对有机阳离子的络合能力更强,而减少桥上碳原子的数量会减弱分子的络合能力.这些新型的弧形分子为构建大环受体提供了很好的骨架单元.
姚欢 , 孙娇囡 , 柯华 , 杨留攀 , 李加荣 , 蒋伟 . 桥联双萘及其衍生物的合成以及对有机阳离子的络合作用[J]. 有机化学, 2017 , 37(3) : 603 -607 . DOI: 10.6023/cjoc201612033
In this research, the structure of the bis-naphthalene molecular resulting from 2-naphthol and 1,1,1',1'-tetramethoxypropane was modified, and six derivatives with different substituents, sidewalls or bridges were synthesized. Their structures were studied by X-ray crystallography and molecular modelling, and all possess curved architectures. Electrostatic potential energy surfaces show that their inner cavities are electron-rich, and may complex with organic cations through cation-π interactions. Their binding stoichiometry and association constants with the 1,4-diazabicyclo[2.2.2]octane (DABCO)-based organic cation were studied by 1H NMR titration and Job's plot. The results show that electron-rich molecules have much stronger association, and reducing the carbon atom in the bridge significantly decreases their association ability. These novel curved structures may work as building blocks for the construction of new macrocyclic receptors.

Key words: host-guest chemistry; macrocyclic chemistry; bis-naphthalene
[1] (a) Tian, J.; Chen, L.; Zhang, D. W.; Liu, Y.; Li, Z. T. Chem. Commun. 2016, 52, 6351.
(b) Chi, X.; Yu, G.; Shao, L.; Chen, J.; Huang, F. J. Am. Chem. Soc. 2016, 138, 3168.
(c) Gao, B.; Tan, L. L.; Song, N.; Li, K.; Yang, Y. W. Chem. Commun. 2016, 52, 5804.
(d) Zhang, W.; Zhang, Y. M.; Li, S. H.; Cui, Y. L.; Yu, J.; Liu, Y. Angew. Chem., Int. Ed. 2016, 128, 11624.
(e) Ma, J.; Meng, Q.; Hu, X.; Li, B.; Ma, S.; Hu, B.; Li, C. Org. Lett. 2016, 18, 5740.
(f) Chen, H.; Fan, J.; Hu, X.; Ma, J.; Wang, S.; Li, J.; Li, C. Chem. Sci. 2015, 6, 197.
(g) Wang, X.; Han, K.; Li, J.; Jia, X.; Li, C. Polym. Chem. 2013, 4, 3998.
(h) Wang, Y.; Ping, G.; Li, C. Chem. Commun. 2016, 52, 9858.
[2] (a) Zhou, C. E.; Zhao, Z. G.; Tang, X. L. Chin. J. Org. Chem. 2007, 27, 513 (in Chinese).(周彩娥, 赵志刚, 唐晓丽, 有机化学, 2007, 27, 513.)
(b) Peng, Y.; Mou, Q. M.; Yang, Z. X.; Chen, S. H. Chin. J. Org. Chem. 2004, 24, 399 (in Chinese).(彭游, 牟其明, 杨祖幸, 陈淑华, 有机化学, 2004, 24, 399.)
[3] (a) Rebek, J. Science 1987, 235, 1478.
(b) Rebek, J. Pure Appl. Chem. 1989, 61, 1517.
[4] Harmata, M. Acc. Chem. Res. 2004, 37, 862.
[5] Dolenský, B.; Havlík, M.; Král, V. Chem. Soc. Rev. 2012, 41, 3839.
[6] (a) Han, T.; Chen, C. F. Org. Lett. 2006, 8, 1069.
(b) Chen, C. F. Chem. Commun. 2011, 47, 1674.
(c) Jiang, Y.; Chen, C. F. Eur. J. Org. Chem. 2011, 32, 6377.
(d) Meng, Z.; Han, Y.; Wang, L. N.; Xiang, J. F.; He, S. G.; Chen, C. F. J. Am. Chem. Soc. 2015, 137, 9739.
[7] Yang, L. P.; Liu, W. E.; Jiang, W. Tetrahedron Lett. 2016, 57, 3978.
[8] (a) Jia, F.; He, Z.; Yang, L. P.; Pan, Z. S.; Yi, M.; Jiang, R. W.; Jiang, W. Chem. Sci. 2015, 6, 6731.
(b) Jia, F.; Wang, H. Y.; Li, D. H.; Yang, L. P.; Jiang, W. Chem. Commun. 2016, 52, 5666.
(c) Jia, F.; Li, D. H.; Yang, T. L.; Yang, L. Pan.; Dang, L.; Jiang, W. Chem. Commun. 2017, 53, 336.
(d) Yang, L. P.; Jia, F.; Zhou, Q. H.; Pan, F.; Sun, J. N.; Rissanen, K.; Chung, L. W.; Jiang, W. Chem.-Eur. J. 2017, 23, 1516.
[9] He, Z.; Yang, X.; Jiang, W. Org. Lett. 2015, 17, 3880.
[10] (a) He, Z.; Ye, G.; Jiang, W. Chem. Eur. J. 2015, 21, 3005.
(b) Huang, G. B.; Jiang, W. Prog. Chem. 2015, 27, 744 (in Chinese).(黄国宝, 蒋伟, 化学进展, 2015, 27, 744.)
[11] (a) Huang, G.; He, Z.; Cai, C. X.; Pan, F.; Yang, D.; Rissanen, K.; Jiang, W. Chem. Commun. 2015, 51, 15490.
(b) Huang, G.; Valkonen, A.; Rissanen, K.; Jiang, W. Chem. Commun. 2016, 52, 9078.
[12] Huang, G. B.; Wang, S. H.; Ke, H.; Yang, L. P.; Jiang, W. J. Am. Chem. Soc. 2016, 138, 14550.
[13] Van Allan, J. A.; Giannini, D. D.; Whitesides, T. H. J. Am. Chem. Soc. 1982, 47, 820.
[14] Shorthill, B. J.; Avetta, C. T.; Glass, T. E. J. Am. Chem. Soc. 2004, 126, 12732.
[15] Geiseler, O.; Müller, M.; Podlech, J. Tetrahedron 2013, 69, 3683.
[16] Kito, T.; Yoshinaga, K.; Yamaye, M.; Mizobe, H. J. Org. Chem. 1991, 56, 3336.
/
| 〈 |
|
〉 |