

多形炭角菌Xylaria polymorpha菌丝发酵的次级代谢产物研究
收稿日期: 2016-12-12
修回日期: 2016-12-22
网络出版日期: 2017-01-04
基金资助
公益性行业(农业)科研专项(No.201303117)、中央级公益性科研院所基本科研业务费(No.1630052016008)、海南省自然科学基金面上(No.20153143)资助项目.
Chemical Constituents from the Cultures of Fungus Xylaria polymorpha
Received date: 2016-12-12
Revised date: 2016-12-22
Online published: 2017-01-04
Supported by
Project supported by the Special Fund for Agro-Scientific Research in the Public Interest (No. 201303117), the Fundamental Scientific Research Funds for Chinese Academy of Tropical Agricultural Sciences (No. 1630052016008) and the Natural Science Foundation of Hainan Province (No. 20153143).
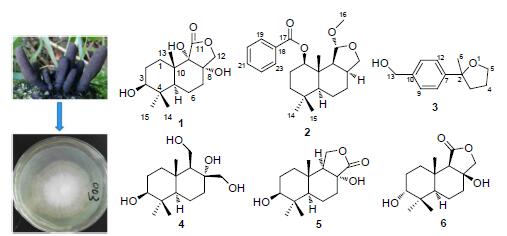
从多形炭角菌Xylaria polymorpha (Pers.:Fr.) Grer菌丝发酵产物乙酸乙酯部分中分离鉴定了21个化合物,包括2个新的drimane型倍半萜polymorphines A,B (1~2),1个新的phenyloxolane类化合物2-甲基-2-(4-羟甲基苯基)氧杂环戊烷(3)和18个已知化合物4~21. 利用NMR和X射线单晶衍射技术对上述化合物结构进行鉴定与确认. 化合物2具有一定的乙酰胆碱酯酶及α-葡萄糖苷酶抑制活性. 此外,化合物3具有中等的全齿复活线虫Panagrellus redivivus抑制活性,在浓度为2.5 mg/mL时,线虫致死率为59.6%.
杨宁宁 , 孔凡栋 , 马青云 , 黄圣卓 , 罗都强 , 周丽曼 , 戴好富 , 郁志芳 , 赵友兴 . 多形炭角菌Xylaria polymorpha菌丝发酵的次级代谢产物研究[J]. 有机化学, 2017 , 37(4) : 1033 -1039 . DOI: 10.6023/cjoc201612039
Totally 21 compounds were isolated from the EtOAc extract of fermentation broth of fungus Xylaria polymorpha (Pers.: Fr.) Grer, including two new drimane-type sesquiterpenoids named polymorphines A and B (1~2) and one new phenyloxolane compound named 2-methyl-2-(4-hydroxymethylphenyl) oxolane (3), together with 18 known compounds 4~21. The structures of these compounds were elucidated by NMR and single-crystal X-ray diffraction analysis. Compound 2 exhibited anti-acetylcholinesterase and α-glucosidase inhibitory activities. Moreover, compound 3 showed moderate inhibitory activitiy against the nematode Panagrellus redivivus with mortality ratio of 59.6% at 2.5 mg/mL.

[1] Lee, I. K.; Jang, Y. W.; Kim, Y. S.; Yu, S. H.; Lee, K. J.; Park, S. M.; Oh, B. T.; Chae, J. C.; Yun, B. S. J. Antibiot. 2009, 62, 163.
[2] Shiono, Y.; Motoki, S.; Koseki, T.; Murayama, T.; Tojima, M.; Kimura, K. I. Phytochemistry 2009, 70, 935.
[3] Jang, Y. W.; Lee, I. K.; Kim, Y. S.; Lee, S. K.; Lee, H. J.; Yu, S. H.; Yun, B. S. J. Antibiot. 2007, 60, 696.
[4] Shan, W.-G.; Chen, X.-X.; Ying, Y.-M.; Zhan, Z.-J. HeIv. Chim. Acta 2011, 94, 1254.
[5] William, A. A.; Latchezar, S. T. J. Nat. Prod. 1992, 55, 1454.
[6] He, J.-B.; Tao, J.; Miao, X.-S.; Bu, W.; Zhang, S.; Dong, Z.-J.; Li, Z.-H.; Feng, T.; Liu, J.-K. Fitoterapia 2015, 102, 1.
[7] Li, Q.-J.; Wang, C.; Li, J.-X.; Zhang, J.-J.; Liu, Y.-H.; Pan, L.-T. Chin. Pharm. J. 2014, 49, 550 (in Chinese).
(李齐激, 王冲, 李继新, 张敬杰, 刘亚华, 潘炉台, 中国药学杂志, 2014, 49, 550.)
[8] Pei, G.; Zhou, P.-H.; He, G.-X.; Du, F.-L.; Jiang, D.-S. Nat. Prod. Res. Dev. 2004, 16, 213 (in Chinese).
(裴刚, 周朴华, 何贵霞, 杜方麓, 蒋道松, 天然产物研究与开发, 2004, 16, 213.)
[9] Dean, V.-J.; Herfried, G. Tetrahedron 1997, 53, 617.
[10] Huang, X.-S.; Gao, S.; Fan, L. H.; Yu, S.-S.; Liang, X.-T. China J. Chin. Mater. Med. 2004, 29, 1108 (in Chinese).
(黄学石, 郜嵩, 范丽华, 庾石山, 梁晓天, 中国中药杂志, 2004, 29, 1108.)
[11] Zhou, W.-W.; Guo, S.-X. Chem. Nat. Compd. 2009, 45, 124.
[12] Li, W.-H.; Chang, S.-T.; Chang, S.-C.; Chang, H.-T. Nat. Prod. Res. 2008, 22, 1085.
[13] Luo, P.; Su, J.; Zhu, Y.-L.; Wei, J.-H.; Wei, W.-X.; Pan, W.-G. Nat. Prod. Res. 2016, 30, 2190.
[14] Hybelbauerová, S.; Sejbal, J.; DracInsky, M.; Hahnová, A.; Koutek, B. Chem. Biodiversity 2008, 5,743.
[15] Liu, X.-H.; Miao, F.-P.; Liang, X.-R.; Ji, N.-Y. Nat. Prod. Res. 2014, 28, 1182.
[16] Kwon, H. C.; Zee, S. D.; Cho, S. Y.; Choi, S. U.; Lee, K. R. Arch. Pharm. Res. 2002, 25, 851.
[17] Gao, H.; Hong, K.; Zhang, X.; Liu, H.-W.; Wang, N.-L.; Zhuang, L.; Yao, X.-S. HeIv. Chim. Acta 2007, 90, 1165.
[18] Fangkrathok, N.; Sripanidkulchai, B.; Umehara, K.; Noguchi, H. Nat. Prod. Res. 2012, 27, 1611.
[19] Amagata, T.; Tanaka, M.; Yamada, T.; Doi, M.; Minoura, K.; Ohishi, H.; Yamori, T.; Numata, A. J. Nat. Prod. 2007, 70, 1731.
[20] E, H.-C.; Zhou, W.; Liu, B.-S.; Tang, H.;Sun, P.; Li, L.; Zhang, W. Chin. J. Marine Drugs 2013, 32, 8 (in Chinese).
(鄂恒超, 周巍, 刘宝姝, 汤华, 孙鹏, 李玲, 张文, 中国海洋药物, 2013, 32, 8.)
[21] Butova, E. D.; Barabash, A. V.; Petrova, A. A.; Kleiner, C. M.; Schreiner, P. R.; Fokin, A. A. J. Org. Chem. 2010, 75, 6229.
[22] Ellman, G. L.; Courtney, K. D.; Jr., V. A.; Featherstone, R. M. Biochem. Pharmacol. 1961, 7, 88.
[23] Li, T.; Zhang, X.-D.; Song, Y.-W.; Liu, J.-W. Chin. J. Clin. Pharmacol. Ther. 2005, 10, 1128 (in Chinese).
(李婷, 张小东, 宋聿文, 刘建文, 中国临床药理学与治疗学, 2005, 10, 1128.)
/
| 〈 |
|
〉 |