

基于咔唑-席夫碱的高选择性裸眼和荧光识别Cu2+的探针
收稿日期: 2016-11-12
修回日期: 2016-12-26
网络出版日期: 2017-01-17
基金资助
辽宁省自然科学基金(No.20102126)资助项目.
Highly Selective Naked-Eye and Fluorescence Probe for Cu2+ Based on Carbazole Schiff-Base
Received date: 2016-11-12
Revised date: 2016-12-26
Online published: 2017-01-17
Supported by
Project supported by the Natural Science Foundation of Liaoning Province (No. 20102126).
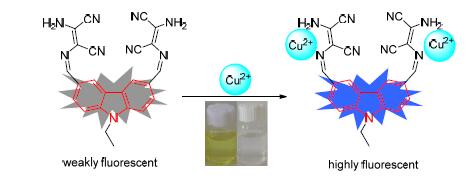
合成了一种新型基于咔唑-席夫碱识别Cu2+的荧光探针L. 利用紫外-可见和荧光光谱研究了探针L对阳离子的识别性能. 实验结果显示,当加入Cu2+时,探针L的CH3CN溶液显示出明显的颜色变化,由黄色变为无色. 这表明,利用探针L可裸眼识别Cu2+. 通过荧光光谱分析实验发现,在CH3CN溶剂中,探针L对Cu2+具有较高的选择性和灵敏度,其荧光强度随着Cu2+的浓度增大而逐渐增强,不受其它金属离子影响,抗干扰能力强. 探针L与Cu2+的结合常数为1.38×104 L/mol,检测限为2.34×10-7 mol/L,低于世界卫生组织(WHO)规定的饮用水中Cu2+的最大含量20 mmol/L. 探针L在检测环境中的Cu2+含量方面具有潜在应用价值.
李英俊 , 李继阳 , 许永廷 , 靳焜 , 曹欣 . 基于咔唑-席夫碱的高选择性裸眼和荧光识别Cu2+的探针[J]. 有机化学, 2017 , 37(4) : 896 -901 . DOI: 10.6023/cjoc201611014
A new fluorescence probe L based on carbazole Schiff-base for Cu2+ was synthesized. The recognition ability of probe L to metal ions was investigated by UV-Vis and fluorescence spectra. The experimental results showed that the probe L in CH3CN displayed a distinct color change from yellow to colorless upon the addition of Cu2+, which can be directly detected by the naked eye for Cu2+. The fluorescence spectra showed that the probe L had high selective and sensitive recognition toward Cu2+ in CH3CN, the fluorescence intensity was increased with the increase of Cu2+ concentration, and it was not affected by other metal ions. The association constant between the probe L and Cu2+ was detected to be 1.38×104 L/mol and the corresponding detection limit was calculated to be 2.34×10-7 mol/L. The detection limit of L for Cu2+ was far lower than the maximum allowable level of the WHO limit (20 mmol/L) for drinking water. The results indicate that the probe L should be applicable to the research on the environmental applications associated with Cu2+.

Key words: carbazole; Schiff base; naked-eye recognition; Cu2+ fluorescence probe
[1] Liu, W. Y.; Li, H. Y.; Lü, H. S.; Zhao, B. X.; Miao, J. Y. Spectrochim. Acta, Part A 2012, 95, 658.
[2] Li, W.; Zhang, Y.; Gan, X. P.; Yang, M. D.; Mie, B.; Fang, M.; Zhang, Q. Y.; Yu, J. H.; Wu, J. Y.; Tian, Y. P.; Zhou, H. P. Sens. Actuators, B: Chem. 2015, 206, 240.
[3] Qin, J. C.; Yang, Z. Y. J. Photochem. Photobiol., A 2016, 324, 152.
[4] Zhang, C. H.; Gao, B. Z.; Zhang, Q. Y.; Zhang, G. M.; Shuang, S. M.; Dong, C. Talanta 2016, 154, 278.
[5] Na, Y. J.; Choi, Y. W.; Yun, J. Y.; Park, K. M.; Chang, P. S.; Kim, C. Spectrochim. Acta, Part A 2015, 136, 1649.
[6] An, R. B.; Zhang, D. T.; Chen, Y.; Cui, Y. Z. Sens. Actuators, B: Chem. 2016, 222, 48.
[7] Yu, H.; Lee, J. Y.; Angupillai, S.; Wang, S.; Feng, S. H.; Matsumoto, S.; Son, Y. A. Photochem. Photobiol., A 2015, 151, 48.
[8] Dusek, P.; Roos, P. M.; Litwin, T.; Schneider, S. A.; Flaten, T. P.; Aaseth, J. J. Trace Elem. Med. Biol. 2015, 31, 193.
[9] Razi, S. S.; Srivastava, P.; Ali R.; Gupta, R. C.; Dwivedi, S. K.; Misra, A. Sens. Actuators, B: Chem. 2015, 209, 162.
[10] Xiong, J. J.; Huang, P. C.; Zhou, X.; Wu, F. Y. Sens. Actuators, B: Chem. 2016, 232, 673.
[11] Li, S.; Zhang, D.; Xie, X. Y.; Ma, S. G.; Liu, Y.; Xu, Z. H.; Gao, Y. F.; Ye, Y. Sens. Actuators, B: Chem. 2016, 224, 661.
[12] Tian, M. Z.; Hu, M. M.; Fan, J. L.; Peng, X. J., Wang, J. Y.; Sun, S. G.; Zhang R. Bioorg. Med. Chem. Lett. 2013, 23, 2916.
[13] Zhu, W. J.; Yang, L. L.; Fang, M.; Wu, Z. Y.; Zhang, Q.; Yin, F. F.; Huang, Q.; Li C. J Fluoresc. 2015, 158, 38.
[14] Danjou, P. E.; Lyskawa, J.; Delattre, F.; Becuwe, M., Woisel, P.; Ruellan, S.; Fourmentin, S.; Dennina, F. C. Sens. Actuators, B: Chem. 2012, 171~172, 1022.
[15] Zhang, J.; Cui, H. L.; Hojo, M.; Shuang, S. M.; Dong, C. Bioorg. Med. Chem. Lett. 2012, 22, 343.
[16] Yang, L. L.; Zhu, W. J.; Fang, M.; Zhang, Q.; Li, C. Spectrochim. Acta, Part A 2013, 109, 186.
[17] Sharma, S.; Pradeep, C. P.; Dhir, A. Mater. Sci. Eng., C 2014, 43, 418.
[18] Feng, G. L.; Geng, L. J.; Wang, T.; Li, J. Y.; Yun, X. D.; Wang, Y. Q.; Li, Y., Xie, D. Y. J Fluoresc. 2015, 167, 65.
[19] Feng, Y.; Li, D. X.; Wang, Q.; Wang, S. X.; Meng, X. M.; Shao, Z. L.; Zhu, M. Z.; Wang, X. Sens. Actuators, B: Chem. 2016, 225, 572.
[20] Li, D. X.; Sun, X.; Huang, J. M.; Wang, Q.; Feng, Y.; Chen, M.; Meng, X. M.; Zhu, M. Z.; Wang, X. Dyes Pigm. 2016, 125, 185.
[21] Kundu, A.; Hariharan, P. S.; Prabakaran, K.; Anthony, S. P. Sens. Actuators, B: Chem. 2015, 206, 524.
[22] Gou, C.; Qin, S. H.; Wu, H. Q.; Wang, Y.; Luo, J.; Liu, X. Y. Inorg. Chem. 2011, 14, 1622.
[23] Chen, H. H.; Guan, R. F.; Cao, D. X.; Liu, Z. Q.; Sun, Y. H.; Ma, L.; Wang, K. N.; Shan, Y. Y. Mater. Lett. 2014, 122, 70.
[24] Wang, W. G.; Li, R.; Song, T. W.; Zhang, C. J.; Zhao, Y. Spectrochim. Acta, Part A 2016, 164, 133.
[25] Velmathi, S.; Reena, V.; Suganya, S.; Anandan, S. J Fluoresc. 2012, 22, 155.
[26] Liu, G.; Shao, J. J Fluoresc. 2012, 22, 397.
[27] Ye, W. P.; Wang, S. X.; Meng, X. M.; Feng, Y.; Sheng, H. T.; Shao, Z. L.; Zhu, M. Z.; Guo, Q. X. Dyes Pigm. 2014, 101, 30.
[28] Wang, L. Y.; Yang, L. L.; Cao, D. J Fluoresc. 2014, 24, 1347.
[29] Kim, H.; Rao, B. A.; Jeong, J. W.; Mallick, S.; Kang, S. M.; Choi, J. S.; Lee, C. S.; Son, Y. A. Sens. Actuators, B: Chem. 2015, 210, 173.
[30] Hammud, H. H.; Shazly, S. E.; Sonji, G.; Sonji, N.; Bouhadir, K. H. Spectrochim. Acta, Part A 2015, 150, 94.
[31] Bu, J.; Duan, H. D.; Wang, X. J.; Xu, T.; Meng, X.; Qin, D. W. Res. Chem. Intermed. 2015, 41, 2767.
[32] Zhu, W. J.; Yang, L. L.; Fang, M.; Wu, Z. Y.; Zhang, Q.; Yin, F. F.; Huang, Q.; Li, C. J. Lumin. 2015, 158, 38.
[33] Liu, W.; Wu, G. Y.; Gu, X. M.; Yuan, X. S.; Li, J. Y.; Wang, H. B. J. Fluoresc. 2015, 25, 557.
[34] Thangavel, S.; Rajamanikandan, R.; Friedrich, H. B.; Ilanchelian, M.; Omondi, B. Polyhedron 2016, 107, 124.
[35] Sharma, S.; Pradeep, C. P.; Dhir, A. Mater. Sci. Eng., C 2014, 43, 418.
[36] Hou, S. H.; Qu, Z. G.; Zhong, K. L.; Bian, Y. J.; Tang, L. J. Chin. J. Org. Chem. 2016, 36, 768 (in Chinese).
(侯淑华, 曲忠国, 钟克利, 边延江, 汤立军, 有机化学, 2016, 36, 768.)
[37] Meng, W. F.; Yang, M. P.; Li, B.; Cheng, Z.; Yang, B. Q. Tetrahedron 2014, 70, 8577.
[38] Jagadeeswari, S.; Paramaguru, G.; Thennarasu, S.; Renganathan, R. J. Mol. Struct. 2014, 1060, 191.
[39] Ramkumar, S.; Manoharan, S.; Anandan, S. Dyes Pigm. 2012, 94, 503.
[40] Budreckiene, R.; Buika, G.; Grazulevicius, J. V.; Jankauskas, V.; Staniskiene, B. J. Photochem. Photobiol., A 2006, 181, 257.
/
| 〈 |
|
〉 |