

石松生物碱集成合成的研究进展
收稿日期: 2016-11-24
修回日期: 2016-12-24
网络出版日期: 2017-01-17
基金资助
国家自然科学基金(Nos.21403169,21502153)、中国博士后科学基金(No.2013M532084)、中央高校基本科研业务费(No.2452015079)资助项目.
Advances in the Collective Synthesis of Lycopodium Alkaloids
Received date: 2016-11-24
Revised date: 2016-12-24
Online published: 2017-01-17
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21403169, 21502153), the Postdoctoral Science Foundation of China (No. 2013M532084) and the Chinese Universities Scientific Fund (No. 2452015079).
肖春霞 , 曹林 , 王佳 , 苗银龙 , 范华芳 . 石松生物碱集成合成的研究进展[J]. 有机化学, 2017 , 37(4) : 810 -823 . DOI: 10.6023/cjoc201611032
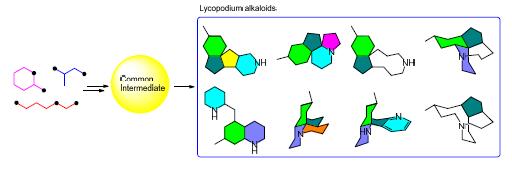
In view of the relevance between chemical structures and biosynthetic pathways of compounds that belong to the same family of natural products, MacMillan et al. (2011) proposed a strategy for the rapid and efficient synthesis of natural product within a family from a common intermediate or a group of similar intermediates, which is termed as collective synthesis. In recent years, this strategy has been applied for the synthesis of multiple family of natural products. Lycopodium alkaloids are a class of structurally diverse alkaloids, and many of them exhibiting good biological activity. In this review, the progress in the collective synthesis of Lycopodium alkaloids is summarized.
[1] (a) Zhao, G.; Dai, S.; Chen, R. Dictionary of Traditional Chinese Medicine, 2 ed., Shanghai Scienfitic & Technical Publishers, Shanghai, 2009, pp. 284~285, 774~775, 1590~1591 (in Chinese).
(赵国平, 戴慎, 陈仁寿, 中药大辞典, 第二版, 上海科学技术出版社, 上海, 2009, pp. 284~285, 774~775, 1590~1591.)
[2] Choo, C. Y.; Hirasawa, Y.; Karimata, C.; Koyama, K.; Sekiguchi, M.; Kobayashi, J. I.; Morita, H. Bioorg. Med. Chem. 2007, 15, 1703.
[3] (a) Mandal, S. K.; Biswas, R.; Bhattacharyya, S. S.; Paul, S.; Dutta, S.; Pathak, S.; Khuda-Bukhsh, A. R. Eur. J. Pharmacol. 2010, 626, 115.
(b) Ham, Y.-M.; Yoon, W.-J.; Park, S.-Y.; Jung, Y.-H.; Kim, D.; Jeon, Y.-J.; Wijesinghe, W.; Kang, S.-M.; Kim, K.-N. Food Chem. Toxicol. 2012, 50, 2629.
[4] He, J.; Chen, X.-Q.; Li, M.-M.; Zhao, Y.; Xu, G.; Cheng, X.; Peng, L.-Y.; Xie, M.-J.; Zheng, Y.-T.; Wang, Y.-P. Org. Lett. 2009, 11, 1397.
[5] Yuan, C.; Chang, C. -T.; Axelrod, A.; Siegel, D. J. Am. Chem. Soc. 2010, 132, 5924.
[6] Feng, X.; Jiang, H.; Zhang, Y.; He, W.; Zhang, L. J. Med. Plants Res. 2012, 6, 1263.
[7] (a) Ayer, W. A. Nat. Prod. Rep. 1991, 8, 455.
(b) Maclean, D. B. In The Alkaloids: Chemistry and Pharmacology, Vol. 26, Ed.: Arnold, B., Academic Press, New York, 1985, Chapter 5.
(c) Luan, X.; Xu, Z. Acta Pharm. Sin. 1986, 21, 310 (in Chinese).
(栾新慧, 徐择邻, 药学学报, 1986, 21, 310.)
(d) Yuan, S.; Luan, X. Bull. Acad. Mil. Med. Sci. 1999, 23, 58 (in Chinese).
(袁珊琴, 栾新慧, 军事医学科学院院刊, 1999, 23, 58.)
(e) Tan, C.; Zhu, D. Chin. J. Nat. Med. 2003, 1, 1 (in Chinese).
(谭昌恒; 朱大元, 中国天然药物, 2003, 1, 1.)
(f) Chen, Y.; Liu, Y.; Jiang, J.; Liu, B. J. Yunnan Normal Univ. (Nat. Sci. Ed.) 2010, 30, 12 (in Chinese).
(陈业高; 刘怡君; 蒋金和; 刘波, 云南师范大学学报(自然科学版), 2010, 30, 12.)
[8] Liu, J.; Yu, C.; Zhou, Y.; Han, Y.; Qi, B.; Zhu, Y. Acta Chim. Sinica 1986, 44, 1035 (in Chinese).
(刘嘉森; 俞超美; 周有作, 化学学报, 1986, 44, 1035.)
[9] (a) Newman, D. J.; Cragg, G. M. J. Nat. Prod. 2012, 75, 311.
(b) Li, J. W. -H.; Vederas, J. C. Science 2009, 325, 161.
[10] (a) Jones, S. B.; Simmons, B.; Mastracchio, A.; MacMillan, D. W. C. Nature 2011, 475, 183.
(b) Schreiber, S. L. Science 2000, 287, 1964.
[11] (a) Yang, H.; Carter, R. G.; Zakharov, L. N. J. Am. Chem. Soc. 2008, 130, 9238.
(b) Yang, H.; Carter, R. G. J. Org. Chem. 2010, 75, 4929.
[12] (a) Saha, M.; Carter, R. G. Org. Lett. 2013, 15, 736.
(b) Saha, M.; Li, X.; Collett, N. D.; Carter, R. G. J. Org. Chem. 2016, 81, 5963.
[13] Ding, R.; Sun, B.-F.; Lin, G.-Q. Org. Lett. 2012, 14, 4446.
[14] (a) Ding, R.; Fu, J.-G.; Xu, G.-Q.; Sun, B.-F.; Lin, G.-Q. J. Org. Chem. 2014, 79, 240.
(b) Fu, J.-G.; Xu, G.-Q.; Ding, R.; Lin, G.-Q.; Sun, B.-F. Org. Chem. Front. 2016, 3, 62.
[15] (a) Heathcock, C. H.; Smith, K. M.; Blumenkopf, T. A. J. Am. Chem. Soc. 1986, 108, 5022.
(b) Heathcock, C. H.; Blumenkopf, T. A.; Smith, K. M. J. Org. Chem. 1989, 54, 1548.
[16] Murphy, R. A.; Sarpong, R. Chem.-Eur. J. 2014, 20, 42.
[17] Nakayama, A.; Kitajima, M.; Takayama, H. Synlett 2012, 23, 2014.
[18] (a) Otsuka, Y.; Inagaki, F.; Mukai, C. J. Org. Chem. 2010, 75, 3420.
(b) Kozaka, T.; Miyakoshi, N.; Mukai, C. J. Org. Chem. 2007, 72, 10147.
(c) Itoh, N.; Iwata, T.; Sugihara, H.; Inagaki, F.; Mukai, C. Chem.-Eur. J. 2013, 19, 8665.
[19] Pan, G.; Williams, R. M. J. Org. Chem. 2012, 77, 4801.
[20] (a) Zaimoku, H.; Taniguchi, T. Chem.-Eur. J. 2014, 20, 9613.
(b) Zaimoku, H.; Nishide, H.; Nishibata, A.; Goto, N.; Taniguchi, T.; Ishibashi, H. Org. Lett. 2013, 15, 2140.
[21] (a) Yang, Y.-R.; Lai, Z.-W.; Shen, L.; Huang, J.-Z.; Wu, X.-D.; Yin, J.-L.; Wei, K. Org. Lett. 2010, 12, 3430.
(b) Yang, Y.-R.; Shen, L.; Wei, K.; Zhao, Q.-S. J. Org. Chem. 2010, 75, 1317.
(c) Yang, Y.-R.; Shen, L.; Huang, J.-Z.; Xu, T.; Wei, K. J. Org. Chem. 2011, 76, 3684.
(d) Dong, L.-B.; Wu, Y.-N.; Jiang, S.-Z.; Wu, X.-D.; He, J.; Yang, Y.-R.; Zhao, Q.-S. Org. Lett. 2014, 16, 2700.
[22] Xu, T.; Luo, X.-L.; Yang, Y.-R. Tetrahedron Lett. 2013, 54, 2858.
[23] Jiang, S.-Z.; Lei, T.; Wei, K.; Yang, Y.-R. Org. Lett. 2014, 16, 5612.
[24] (a) Zhang, X.-M.; Tu, Y.-Q.; Zhang, F.-M.; Shao, H.; Meng, X. Angew. Chem., Int. Ed. 2011, 50, 3916.
(b) Zhang, X.-M.; Shao, H.; Tu, Y.-Q.; Zhang, F.-M.; Wang, S.-H. J. Org. Chem. 2012, 77, 8174.
[25] (a) Li, H.; Wang, X.; Lei, X. Angew Chem., Int. Ed. 2012, 51, 491.
(b) Li, H.; Wang, X.; Hong, B.; Lei, X. J. Org. Chem. 2013, 78, 800.
(c) Wang, X.; Li, H.; Lei, X. Synlett 2013, 24, 1032.
(d) Zhang, J.; Wu, J.; Hong, B.; Ai, W.; Wang, X.; Li, H.; Lei, X. Nat. Commun. 2014, 5, 4614.
(e) Hong, B.; Li, H.; Wu, J.; Zhang, J.; Lei, X. Angew. Chem., Int. Edit. 2015, 54, 1011.
[26] (a) Zeng, C.; Zheng, C.; Zhao, J.; Zhao, G. Org. Lett. 2013, 15, 5846.
(b) Zeng, C.; Zhao, J.; Zhao, G. Tetrahedron 2015, 71, 64.
[27] Hou, S.-H.; Tu, Y.-Q.; Liu, L.; Zhang, F.-M.; Wang, S.-H.; Zhang, X.-M. Angew. Chem., Int. Ed. 2013, 52, 11373.
[28] Wolfe, B. H.; Libby, A. H.; Al-Awar, R. S.; Foti, C. J.; Comins, D. L. J. Org. Chem. 2010, 75, 8564.
[29] Shigeyama, T.; Katakawa, K.; Kogure, N.; Kitajima, M.; Takayama, H. Org. Lett. 2007, 9, 4069.
[30] Tanaka, T.; Kogure, N.; Kitajima, M.; Takayama, H. J. Org. Chem. 2009, 74, 8675.
[31] (a) Bradshaw, B.; Luque-Corredera, C.; Bonjoch, J. Org. Lett. 2013, 15, 326.
(b) Bradshaw, B.; Luque-Corredera, C.; Bonjoch, J. Chem. Commun. 2014, 50, 7099.
(c) Bosch, C.; Bradshaw, B.; Bonjoch, J.; Fiser, B.; Gomez-Bengoa, E. Org. Lett. 2015, 17, 5084.
[32] Nishikawa, Y.; Kitajima, M.; Kogure, N.; Takayama, H. Tetrahedron 2009, 65, 1608.
[33] Liau, B. B.; Shair, M. D. J. Am. Chem. Soc. 2010, 132, 9594.
[34] Lee, A. S.; Liau, B. B.; Shair, M. D. J. Am. Chem. Soc. 2014, 136, 13442.
/
| 〈 |
|
〉 |