

盐酸黄连素的汇聚式合成研究
收稿日期: 2016-12-02
修回日期: 2017-01-01
网络出版日期: 2017-01-17
基金资助
西南民族大学研究生创新(No.CX2015SZ045)和四川省教育厅(No.15ZB0487)资助项目.
A Concisely Convergent Synthesis of Berberine Chloride
Received date: 2016-12-02
Revised date: 2017-01-01
Online published: 2017-01-17
Supported by
Project supported by the Graduate Innovative Research Projects of Central Universities, Southwest University for Nationalities (No. CX2015SZ045) and the Research Projects Sichuan Provincial Department of Education (No. 15ZB0487).
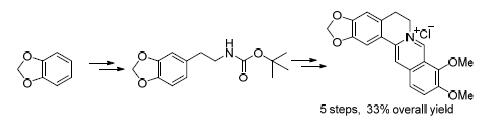
报道了以1,2-亚甲二氧基苯为起始原料合成盐酸黄连素的汇聚式路线,该路线包括格氏试剂亲核开环、脱保护、环合等5步反应,总收率为33%.该路线反应条件温和、操作简单,且所有中间体及产物都经过1H NMR、13C NMR、MS鉴定.
关键词: 盐酸黄连素; 汇聚式合成; 1,2-亚甲二氧基苯; 格氏试剂
陈程 , 徐蒙蒙 , 赵青 , 刘承秀 , 杨鸿均 , 冯豫川 . 盐酸黄连素的汇聚式合成研究[J]. 有机化学, 2017 , 37(2) : 503 -507 . DOI: 10.6023/cjoc201612005
The berberine chloride was synthesized starting from benzo[d][1,3]dioxole with the mild condition and the simple operation in overall 33% yield. The route includes the five steps of nucleophilic ring opening, deprotection and cyclization and so on. All intermediates were determined by 1H NMR, 13C NMR and MS techniques.

[1] (a) Lo, C. Y.; Hsu, L. C.; Chen, M. S.; Lin, Y. J.; Chen, L. G.; Kuo, C. D.; Wu., J. Y. Bioorg. Med. Chem. Lett. 2013, 23, 305.
(b) Li, Q.; Xiang, J. F.; Tang, Y. L. Chin. J. Chem. 2015, 33, 1041.
[2] Zhang, S. L.; Chang, J. J.; Damu, G. V.; Fang, B.; Zhou, X. D.; Geng, R. X.; Zhou, C. H. Bioorg. Med. Chem. Lett. 2013, 23, 1008.
[3] (a) Samosorn, S.; Tanwirat, B.; Muhamad, N.; Casadei, G.; Tomkiewicz, D.; Lewis, K.; Suksamrarn, A.; Prammananan, T.; Gornall, K. C.; Beck, J. L.; Bremner, J. B. Bioorg. Med. Chem. 2009, 17, 3866.
(b) Zhang, S. L.; Damu, G. L.; Zhang, L.; Geng, R. X.; Zhou, C. H. Eur. J. Med. Chem. 2012, 55, 164.
[4] Bodiwala, H. S.; Sabde, S.; Mitra, D.; Bhutani, K. K.; Singh, I. P. Eur. J. Med. Chem. 2011, 46, 1045.
[5] Vennerstrom, J. L.; Lovelace, J. K.; Wsits, V. B.; Hanson, W. L.; Klayman, D. L. Antimicrob. Agents Chemother. 1990, 34, 918.
[6] Bahar, M.; Deng, Y.; Zhu, X.; He, S.; Pandharkar, T.; Drew, M. E.; Navarro-Vazquez, A.; Anklin, C.; Gil, R. R.; Dos Kotch, R. W.; Werbovetz, K. A.; Kinghorn, A. D. Bioorg. Med. Chem. Lett. 2011, 21, 2606.
[7] (a) Letasiova, S.; Jantova, S.; Cipak, L.; Muckova, M. Cancer Lett. 2006, 239, 254.
(b) Ma, Y.; Ou, T. M.; Tan, J. H.; Hou, J. Q.; Huang, S. L.; Gu, L. Q.; Huang, Z. S. Bioorg. Med. Chem. Lett. 2009, 19, 3414.
[8] (a) Decker, V. I. Justus Liebigs Ann. Chem. 1913, 395, 295.
(b) Kametani, T.; Noguchi, l. J. Chem. Soc. (C) 1969, 2036.
(c) Nanning Pharmaceutical Chin. J. Pharm. 1973, (7), 1 (in Chinese).(广西南宁制药厂, 医药工业, 1973, (7), 1.)
(d) Masayuki, O.; Kumiko, Y.; Junko, O. Chem. Pharm. Bull. 1974, 22, 2365.
(e) Hangzhou First Pharmaceutical Reagent Chamber. Chin. J. Pharm. 1974, (8), 6 (in Chinese).(杭州第一制药厂试剂室, 医药工业, 1974, (8), 6.)
(f) North-east Pharmaceutical Factory Chin. J. Pharm. 1975, (4), 12 (in Chinese).(东北制药总厂, 医药工业, 1975, (4), 12.)
(g) Vinogradova, V. I.; Yunusov, M. S.; Kuchin, A. V.; Tolstikov, G. A.; Sagandykov, R. T.; Khaimuratov, K. A.; Ali mov, A. Chem. Nat. Compd. 1990, 26, 54.
(h) Hisashi, I.; Mayumi, O.; Shuji, O.; Takashi, H.; Tsutomu, I. Heterocycles 1994, 37, 897.
(i) Matulenko, M. A.; Meyers, A. I. J. Org. Chem. 1996, 61, 573.
(j) Yang, P.; Song, D. Q.; Li, Y. H.; Kong, W. J.; Wang, Y. X.; Gao, L. M.; Liu, S. Y.; Cao, R. Q.; Jiang, J. D. Bioorg. Med. Chem. Lett. 2008, 18, 4675.
(k) Gatland, A. E.; Pilgrim, B. S.; Procopiou, P. A.; Donohoe, T. J. Angew. Chem. Int. Ed. 2014, 53, 14555.
(l) He, Y.; Zheng, Y.; Hai, L.; Wu, Y. Chin. J. Chem. 2014, 32, 1121.
(m) Reddy, V.; Jadhav, A. S.; Vijaya Anand, R. Org. Biomol. Chem. 2015, 13, 3732.
(n) Chen, C.; Luo, Z. M.; Yang, H. J.; Feng, Y. C. Chin. J. Org. Chem. 2016, 36, 1426 (in Chinese).(陈程, 罗卓玛, 杨鸿均, 冯豫川, 有机化学, 2016, 36, 1426.)
[9] (a) Roy, S. C.; Guin, C.; Rana, K. K.; Maiti, G. Tetrahedron Lett. 2001, 42, 6941.
(b) Roy, S. C.; Guin, C.; Maiti, G. Tetrahedron Lett. 2001, 42, 9253.
[10] Tietze, L. F., Schirok, H. J. Am. Chem. Soc. 1999, 121, 10264.
[11] Pan, J. F.; Yu, C.; Zhu, D. Y.; Zhang, H.; Ren, J. Y. CN 1314347, 2001 [Chem. Abstr. 2002, 137, 370266].
/
| 〈 |
|
〉 |