

新型5,6-二氢-6-烷基吡喃酮类衍生物的设计、合成及肿瘤细胞增殖抑制活性研究
收稿日期: 2016-12-02
修回日期: 2016-12-30
网络出版日期: 2017-01-17
基金资助
河南省高校科技创新人才计划(No.14HASTIT0031)资助项目.
Design, Synthesis and Anti-proliferative Activity of Novel 5,6-Dihydro-6-alkyl-2-pyrone Analogues
Received date: 2016-12-02
Revised date: 2016-12-30
Online published: 2017-01-17
Supported by
Project supported by the Program for Science & Technology Innovation Talents of Henan Province (No. 14HASTIT0031).
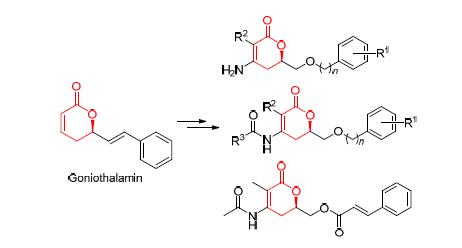
为了研究吡喃酮结构中不饱和双键修饰对该类化合物生物活性的影响. 基于5,6-二氢-6-烷基吡喃酮类化合物哥纳香甲素,通过不饱和双键的修饰,设计合成了化合物5,6和8. 利用噻唑蓝(MTT)法评价了目标化合物对人肝癌细胞(7721)、人肺癌细胞(A549)、人食管癌细胞(EC-109)、人胃癌细胞(MGC-803)的细胞增殖抑制活性. 通过和阳性药物的对比发现,除了4-(2,3,4,5-四氟)苯甲酰氨基-3-甲基-6-苄氧甲基-5,6-二氢-吡喃-2-酮(6d),所合成化合物的肿瘤细胞增殖抑制活性均消失. 结果表明,在5,6-二氢-6-烷基吡喃酮类化合物结构中,不饱和双键起着至关重要的作用,对其进行适当的修饰,在保留其活性的同时,具有潜在改善其因共价键结合引起毒性的可能性.
徐海伟 , 贾世龙 , 谢晓平 , 罗姣 , 王树 . 新型5,6-二氢-6-烷基吡喃酮类衍生物的设计、合成及肿瘤细胞增殖抑制活性研究[J]. 有机化学, 2017 , 37(4) : 902 -907 . DOI: 10.6023/cjoc201612007
In order to investigate whether the modification at the double bonds in the pyrone skeleton would affect their biological activity. Based on the 5,6-dihydro-6-alkyl-2-pyrone goniothalamin, a series of new compounds 5, 6 and 8 were designed and synthesized, which were obtained by modification at the unsubstituted double bonds. Their cell proliferation inhibition activities against human liver cancer (7721), human lung cancer (A549), human esophageal cancer (EC-109), human gastric cancer (MGC-803) cell lines were assessed by thiazolyl blue tetrazolium bromide (MTT) method. Most of synthetic compounds lost their bioactivities via a comparision with positive control but 6-(benzyloxy)methyl-3-methyl-4-(2,3,4,5-tetra- fluorobenzamine)-5,6-dihydro-2H-pyran-2-one (6d). The results indicate that the unsaturated double bond is a key functional group for the bioactivity of these compounds and it is possible to keep their anti-cancer proliferation activity and reduce their toxicity by modification of the unsaturated double bond in 5,6-dihydro-6-alkyl-2-pyrones.

Key words: α,β-unsaturated δ-lactone; synthesis; cancer; antiproliferative activity
[1] Jong, R.; Mulder, N.; Uges, D. Brit. J. Cancer. 1999, 79, 882.
[2] Barros, M. E.; Freitas, J. C.; Oliveira, J. M. Eur. J. Med. Chem. 2014, 76, 291.
[3] Seyed, M. A.; Jantan, I.; Bukhari, S. N. A. BioMed Res. Int. 2014, 2014, 536508.
[4] Sun, Q. X.; Carrasco, Y. P.; Hu, Y. C. P. Natl. Acad. Sci. U. S. A. 2013, 110, 1303.
[5] Sandra, T. G.; Concepcion, V.; Santiago, D. O. Arch. Pharm. Chem. Life Sci. 2015, 348, 541.
[6] Raju, A.; Reddy, A. Y.; Sabitha, G. Tetrahedron Lett. 2015, 56, 5474.
[7] Luiz, F. T. N.; Carolina, M. A.; Karin, J. P. R. Chem. Med. Chem. 2015, 10, 1687.
[8] Vilanova, C.; Diaz-Oltra, S.; Murga, J. J. Med. Chem. 2014, 57, 10391.
[9] Marucci, C.; Christodoulou, M. S.; Pieraccini, S. Eur. J. Org. Chem. 2016, 11, 2029.
[10] Benedekovic, G.; Kovacevic, I.; Popsavin, M. Bioorg. Med. Chem. Lett. 2016, 26, 3318.
[11] Li, X. C.; Guo, Z. Y.; Deng, Z. S. Rec. Nat. Prod. 2015, 9, 503.
[12] He, H. Y.; Ratnayake, A. S.; Janso, J. E. J. Nat. Prod. 2014, 77, 1864.
[13] Marco, J. A.; García-Pla, J.; Carda, M. Eur. J. Med. Chem. 2011, 46, 1630.
[14] Kim, J. H.; Lee, S. J.; Org. Lett. 2011, 13, 1350.
[15] Kim, J. H.; Shin, H.; Lee, S. G. J. Org. Chem. 2012, 77, 1560.
[16] Cao, X. F.; Sun, T. T.; Ke, S. Y. Chin. J. Org. Chem. 2010, 30, 1113 (in Chinese).
(曹秀芳, 孙婷婷, 柯少勇, 有机化学, 2010, 30, 1113.)
[17] Koziol, A.; Lendzion-Paluch, A.; Manikowski, A. Org. Proc. Res. Dev. 2013, 17, 869.
[18] Zhao, J. Y.; Zheng, Z. H.; Huang, Q. F.; Deng, J. G.; Zhu, J.; Wang, Q. W. Chin. J. Org. Chem. 2016, 36, 648 (in Chinese).
(赵剑阳, 郑紫华, 黄晴菲, 邓金根, 朱槿, 王启卫, 有机化学, 2016, 36, 648.)
[19] Pospisil, J.; Marko, I. E. J. Am. Chem. Soc. 2007, 129, 3516.
[20] Ramesh, D.; Rajaram, S.; Peddikotla, P. Helv. Chim. Acta 2011, 94, 1226.
[21] AnkiReddy, S.; AnkiReddy, P.; Sabitha, G. Org. Biomol. Chem. 2015, 13, 10487.
/
| 〈 |
|
〉 |