

离子液体中卤代炔烃高效水解制取α-卤代甲基酮的研究
收稿日期: 2016-10-18
修回日期: 2016-12-30
网络出版日期: 2017-01-20
基金资助
湖南省科技厅基金(No.2015WK3003)、湖南省教育厅科研基金(No.14C0405)资助项目.
Efficient Hydrolysis of Haloalkynes to α-Haloketones in Ionic Liquid
Received date: 2016-10-18
Revised date: 2016-12-30
Online published: 2017-01-20
Supported by
Project supported by the Planned Science and Technology Project of Hunan Province (No. 2015WK3003), the Scientific Research Fund of Hunan Provincial Education Department (No. 14C0405).
付文强 , 谭平 , 邓伟 , 向建南 . 离子液体中卤代炔烃高效水解制取α-卤代甲基酮的研究[J]. 有机化学, 2017 , 37(6) : 1501 -1505 . DOI: 10.6023/cjoc201610031
The haloalkynes were hydrolyzed to α-haloketones by sulfuric acid promotion in ionic liquids (ILs) with 85%~94% yields. The ILs-H2SO4 reaction system could be easily recycled (more five times) without any effect for reaction yield. At the same time, a wide scope of substrates haloalkynes were proper in this reaction system and a series of α-chloro/bromo/iodo acetophenones with different substituent (such as methyl, methoxyl, hydroxyl, nitro etc.) and aliphatic α-haloketones have been obtained in good yields.
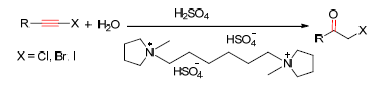
Key words: ionic liquid; haloalkynes; hydration; α-haloketones; recyclable
[1] (a) Erian, A. W.; Sherif, S. M.; Gaber, H. M. Molecules 2003, 8, 793.
(b) Zhang, H.; Wei, Q.; Wei, S.; Qu, J.; Wang, B. Eur. J. Org. Chem. 2016, 3373.
[2] De Kimpe, N.; Verhé, R. The Chemistry of α-Haloketones, α-Haloaldehydes and α-Haloimines, Ed.: Patai, S., Wiley, Chichester, UK, 1988, pp. 1~119.
[3] (a) Ostrowski, T.; Golankiewicz, B.; De Clercq, E.; Andrei, G.; Snoeck, R. Eur. J. Med. Chem. 2009, 44,3313.
(b) Conde, S.; Pérez, D. I.; Martínez, A.; Perez, C.; Moreno, F. J. J. Med. Chem. 2003, 46, 4631.
[4] (a) Morton, H. E.; Leanna, M. R. Tetrahedron Lett. 1993, 34, 4481.
(b) Patil, R. D.; Joshi, G.; Adimurthy, S.; Ranu, B. C. Tetrahedron Lett. 2009, 50, 2529.
(c) Tanemura, K.; Suzuki, T.; Nishida, Y.; Satsumabayashi, K.; Horaguchi, T. Chem. Commun. 2004, 470.
(d) Meshram, H. M.; Reddy, P. N.; Vishnu, P.; Sadashiv, K.; Yadav, J. S. Tetrahedron Lett. 2006, 47, 991.
(e) Pravst, I.; Zupan, M.; Stavber, S. Tetrahedron 2008, 64, 5191.
(f) Kosower, E. M.; Wu, G. S. J. Org. Chem. 1963, 28, 633.
(g) Kajigaeshi, S.; Kakinami, T.; Moriwaki, M.; Fujisaki, S.; Maeno, K.; Okamoto, T. Synthesis 1988, 545.
(h) Dieter, R. K.; Nice, L. E.; Velu, S. E. Tetrahedron Lett. 1996, 37, 2377.
[5] Xie, L.; Wu, Y.; Yi, W.; Zhu, L.; Xiang, J.; He, W. J. Org. Chem. 2013, 78, 9190.
[6] Zou, H.; He, W.; Dong, Q.; Wang, R.; Yi, N.; Jiang, J.; Peng, D.; He, W. Eur. J. Org.Chem. 2016, 116.
[7] (a) Chen, Z.; Ye, D.; Ye, M.; Zhou, Z.; Li, S.; Liu, L. Tetrahedron Lett. 2014, 55, 1373.
(b) Zeng, M.; Huang, R.; Li, W.; Liu, X.; He, F.; Zhang, Y.; Xiao, F. Tetrahedron Lett. 2016, 72, 3818.
[8] (a) Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Appl. Catal. A: Gen. 2010, 373, 1.
(b) Hu, Y.; Guo, Z.; Lue, B. M.; Xu, X. J. Agric. Food Chem. 2009, 57, 3845.
(c) Tzani, A.; Douka, A.; Papadopoulos, A.; Pavlatou, E. A.; Voutsas, E.; Detsi, A. ACS Sustainable Chem. Eng. 2013, 1, 1180.
[9] Wong, W.; Ho, K.; Lee, L.; Lam, K.; Zhou, Z.; Chan, T.; Wong, K. ACS Catal. 2011, 1, 116.
[10] Wei, Q. L.; Zhang, S. S.; Gao, J.; Li, W. H.; Xu, L. Z.; Yu, Z. G. Bioorg. Med. Chem. 2006, 14, 7146.
[11] Reddy Bodireddy, M.; Gangi, R. N. C. Synth. Commun. 2013, 43, 2603.
[12] Guan, X.; AlMisbaa, Z.; Huang, K. Arabian J. Chem. 2015, 8, 892.
[13] Sugiura, A.; Kepner, R. E.; Webb, A. D. J. Org. Chem. 1962, 27, 773.
[14] Jiang, Q.; Sheng, W.; Guo, C. Green Chem. 2013, 15, 2175
[15] Rosenmund, K. W. Chem. Ber. 1957, 90, 1922.
[16] Lapointe, D.; Markiewicz, T.; Whipp, C. J.; Toderian, A.; Fagnou, K. J. Org. Chem. 2011, 76,749.
[17] Judefind, W. L. J. Am. Chem. Soc. 1920, 42, 1043.
[18] Arrieta, A. Synth. Commun. 1984, 14, 939.
[19] Suzuki, M. Yakugaku Zasshi 1952, 72, 305.
[20] Antunes, H.; Fardelone, L. C.; Rodrigues, J. A. R.; Moran, P. J. S. Tetrahedron: Asymmetry 2004, 15, 2615.
/
| 〈 |
|
〉 |