

高圣草素-7-O-β-D-葡萄糖苷及其非对映异构体的合成
收稿日期: 2016-12-05
修回日期: 2017-01-03
网络出版日期: 2017-01-20
基金资助
上海市生物医药领域科技支撑计划(No.15431902700)资助项目.
Synthesis of Homoeriodictyol-7-O-β-D-glycoside and Its Diastereoisomer
Received date: 2016-12-05
Revised date: 2017-01-03
Online published: 2017-01-20
Supported by
Project supported by the Shanghai Biomedical Science and Technology Support Project (No. 15431902700).
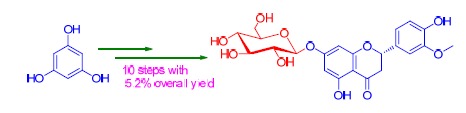
高圣草素-7-O-β-D-葡萄糖苷作为传统中药槲寄生的有效成分已被广泛研究,以间苯三酚、D-葡萄糖为原料,经傅克酰基化、选择性羟基保护、羟醛缩合、相转移催化下的糖苷化等反应步骤首次实现高圣草素-7-O-β-D-葡萄糖苷及其非对映异构体的合成,有效解决提取困难的问题,为其进行相关药理研究奠定物质基础.所合成化合物结构经核磁共振氢谱、碳谱和高分辨质谱确证.
关键词: 槲寄生; 高圣草素-7-O-β-D-葡萄糖苷; 非对映异构体; 合成
康满满 , 马志龙 , 刘彪 , 潘登 , 李建其 . 高圣草素-7-O-β-D-葡萄糖苷及其非对映异构体的合成[J]. 有机化学, 2017 , 37(6) : 1516 -1522 . DOI: 10.6023/cjoc201612012
As the active component in visci herba of traditional Chinese medicine, homoeriodictyol-7-O-β-D-glycoside has been widely studied for years. In this paper, the first synthesis of homoeriodictyol-7-O-β-D-glycoside and its diastereoisomer has been achieved, which was carried out using phloroglucinol and D-glucose as the starting materials through Friedel-Crafts acylation, selective hydroxy protecting, aldol condensation, glycosylation under phase transfer catalytic condition and other reactions. Our work laid the foundation for large scale preparation of homoeriodictyol-7-O-β-D-glycoside instead of inefficient extration, and provided material baisis for furthur pharmaceutical investigation. The structures of synthetic compounds were confirmed by 1H NMR, 13C NMR and HRMS spectra.

Key words: visci herba; homoeriodictyol-7-O-β-D-glycoside; diastereoisomer; synthesis
[1] Cao, D.; Weng, Z.-J.; Li, J.-Q.; Yang, P.-M.; He, Q.-Q.; Cheng, L.; Kong, D.-Y. Chin. Tradit. Herb. Drugs 2015, 46, 1562 (in Chinese). (曹朵, 翁志洁, 李建其, 杨培明, 何泉泉, 成亮, 孔德云, 中草药, 2015, 46, 1562.)
[2] Shanghai Viscum Group. Chin. J. Pharm. 1977, 3, 39 (in Chinese). (上海槲寄生研究协作组, 中国医药工业杂志, 1977, 3, 39.)
[3] Kong, D.-Y.; Luo, S.-Q.; Li, H.-T.; Lei, X.-H. Acta Pharm. Sin. 1988, 23, 593 (in Chinese).(孔德云, 罗思齐, 李惠庭, 雷兴翰, 药学学报, 1988, 23, 593.)
[4] Yang, L.-Y.; Lin, J.; Zhou, B.; Liu, Y.-G.; Zhu, B.-Q. Nat. Prod. Res. 2016, 25, 1.
[5] Zhao, Y.-L.; Ma, M.-Y.; Gao, X.-X.; Liu, T.; Yu, Z.-G.; Bi, K.-S. Chin. J. Chromatogr. 2006, 24, 479 (in Chinese).(赵云丽, 马铭研, 高晓霞, 刘涛, 于志国, 毕开顺, 色谱, 2006, 24, 479.)
[6] Wu, L.; Luo, J.; Zhang, Y.-L.; Zhu, M.-D.; Wang, X.-B.; Luo, J.-G.; Yang, M.-H.; Yu, B.-Y.; Yao, H.-Q.; Dai, Y.; Guo, Q.-L.; Chen, Y.-J.; Sun, H.-B.; Kong, L.-Y. Tetrahedron Lett. 2015, 56, 229.
[7] Zhang, B.-X.; Duan, D.-Z.; Ge, C.-P.; Yao, J.; Liu, Y.-P.; Li, X.-M.; Fang, J.-G. J. Med. Chem. 2015, 58, 1795.
[8] Van, S. N.; Dong, L.-P.; Wang, S.-C.; Wang, Q.-A. Eur. J. Org. Chem. 2015, 10, 2297
[9] Chao, S.-W.; Su, M.-Y.; Chiou, L.-C.; Chen, L.-C.; Chang, C.-I; Huang, W.-J. J. Nat. Prod. 2015, 78, 1969.
[10] Yang, J.-H.; Zuo, W.-B.; Guo, D.-D.; Luo, J.-S.; Huang, W.-Q. Chin. Chem. Lett. 2012, 23, 1375.
[11] Zalihe, H.; Benjamin, C.; Allison, M. H.; Jason, Q. D. G.; Ian, E. W.; Spencer, J. W. Carbohydr. Res. 2010, 345, 2079.
[12] Cai, S.-L.; Wu, Z.; Wu, J.; Wang, Q.-A.; Shan, Y. Chin. J. Org. Chem. 2012, 32, 560 (in Chinese).(蔡双莲, 吴峥, 吴进, 汪秋安, 单杨, 有机化学, 2012, 32, 560.)
[13] Wang, Q.-A.; Wu, Z.; Liu, L.; Zou, L.-H.; Luo, M. Chin. J. Org. Chem. 2010, 30, 1682 (in Chinese). (汪秋安, 吴峥, 刘莉, 邹亮华, 罗茗, 有机化学, 2010, 30, 1682.)
/
| 〈 |
|
〉 |