

微波辅助下高效合成功能性6-芳基水杨酸类衍生物(英文)
收稿日期: 2016-12-19
修回日期: 2017-01-15
网络出版日期: 2017-01-20
基金资助
公益性行业(农业)科研专项经费(No.201203022)、国家科技攻关计划(No.2014BAD23B01)和国家自然科学基金(No.21372093)资助项目.
An Efficient Synthesis of Functionalized 6-Arylsubstituted Salicylates via Microwave Irradiation
Received date: 2016-12-19
Revised date: 2017-01-15
Online published: 2017-01-20
Supported by
Project supported by the Special Fund for Agroscientific Research in the Public Interest (No. 201203022), the National Key Technologies R&D Program (No. 2014BAD23B01) and the National Natural Science Foundation of China (No. 21372093).
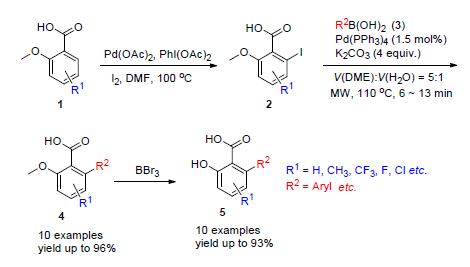
功能化的6-芳基水杨酸片段广泛地存在于各种天然产物以及活性分子中.在基于杂草抗性的分子设计中,6-芳基取代的水杨酸衍生物作为反抗性乙酰羟酸合成酶抑制剂的关键药效团发挥着至关重要的作用.在前期工作中,探索了两种策略可以合成6-芳基取代水杨酸片段,但不能有效地实现在6-芳基水杨酸片段母核结构中的底物多样化.因此,通过微波辅助的Suzuki偶联反应,成功合成了多取代的6-芳基水杨酸片段.这一方法具有反应时间短、反应收率高等优点,并且实现了底物多样化,为后期合成具有反抗性乙酰羟酸合成酶抑制剂奠定了基础.
关键词: 功能化6-芳基水杨酸; Suzuki交叉偶联反应; 微波辐射; 电子效应
曲仁渝 , 陈念 , 刘玉超 , 陈琼 , 杨光富 . 微波辅助下高效合成功能性6-芳基水杨酸类衍生物(英文)[J]. 有机化学, 2017 , 37(5) : 1266 -1272 . DOI: 10.6023/cjoc201612049
Functionalized 6-arylsalicylate substructures occur in a variety of pharmacologically relevant natural products and bioactive compounds. Especially 6-arylsubstituted salicylates, as a key pharmacophore of anti-resistant acetohydroxyacid synthase (AHAS) inhibitors have played a lead role in combatting the weed-resistance issues. Previously, we have explored two new methods to synthesize position-6 aryl substituted salicylic acid fragment. However, these two methods failed to introduce various substituents into salicylic acid. Here an efficient method for the synthesis of 6-substituted salicylates is described via a microwave-promoted Suzuki cross-coupling. Due to the obvious advantages of this method, such as a wide range of substrates, smooth and rapid reaction and moderate to excellent yields, this protocol could be utilized to synthesize more anti-resistant AHAS inhibitors.

[1] (a) Namki, C.; Hee, J. Y.; Hong, P. K.; Sang, H. S. J. Nat. Prod. 2013, 76, 2291.
(b) Gang, S.; Wang, M. W.; Welch, T. R.; Blagg, B. S. J. J. Org. Chem. 2006, 71, 7618.
(c) Delgado, E. J. J. Mol. Model. 2010, 16, 1421.
(d) Abe, H.; Nishioka, K.; Takeda, S.; Arai, M.; Takeuchi, Y.; Harayama, T. Tetrahedron Lett. 2005, 46, 3197.
(e) Lin, C. N.; Huang, P. L.; Lu, C. M.; Yen, M. H., Wu, R. R. Phytochemistry 1997, 44, 1359.
(f) Yang, G. F.; Yang, H. Z. J. Cent. China Norm. Univ., Nat. Sci. 2001, 35, 40 (in Chinese). (杨光富, 杨华铮, 华中师范大学学报(自然科学版), 2001, 35, 40.)
(g) Yang, G. F.; Liu, H. Y.; Lu, R. J.; Yang, H. Z. Chem. J. Chin. Univ. 1998, 19, 222 (in Chinese). (杨光富, 刘华银, 陆荣键, 杨华铮, 高等学校化学学报, 1998, 19, 222.)
[2] (a) He, Y. Z.; Li, Y. X.; Zhu, X. L.; Yang, G. F. J. Chem. Inf. Model. 2007, 47, 2335.
(b) Li, Y. X.; Luo, Y. P.; Xi, Z.; Yang, G. F. J. Agric. Food Chem. 2006, 54, 9135.
(c) Chen, C. N.; Chen, Q.; Liu, Y. C.; Yang, G. F. Bioorg. Med. Chem. 2010, 18, 4897.
(d) Xiong, Y.; Liu, J. J.; Yang, G. F. J. Comput. Chem. 2010, 31, 1592.
(e) Chen, C. N.; Lv, L. L.; Ji, F. Q.; Yang, G. F. Bioorg. Med. Chem. 2009, 17, 3011.
[3] (a) Ji, F. Q.; Niu, C. W.; Yang, G. F.; Xi, Z. Chem. Med. Chem. 2008, 3, 1203.
(b) Liu, Y. C.; Qu, R. Y.; Chen, Q.; Yang, J. F.; Niu, C. W.; Xi, Z.; Yang, G. F. J. Agric. Food Chem. 2016, 64, 4845.
(c) Yang, G. F.; Liu, Y. C.; Chen, Q.; Xi, Z.; Niu, C. CN 104650084, 2015 [Chem. Abstr. 2015, 903779].
(d) Yang, G. F.; Liu, Y. C.; Chen, Q. CN 104140397, 2013
[Chem. Abstr. 2014, 1908130].
[4] (a) Chan, T. H.; Brownbridge, P. J. Am. Chem. Soc. 1980, 102, 3534.
(b) Brownbridge, P.; Chan, T. H.; Brook, M. A.; Kang, G. J. Can. J. Chem. 1983, 61, 688.
(c) Feist, H.; Langer, P. Synthesis 2007, 3, 327.
(d) Stefan, B.; Nazken, K. K.; Zharylkasyn, A. A.; Alexander, V.; Peter, L. Tetrahedron 2012, 68, 3654.
(e) Matthias, L.; Muhammad, S.; Alexander, V.; Christine, F.; Peter, L. Eur. J. Org. Chem. 2010, 5118.
(f) Mohanad, S.; Olumide, F.; Abdolmajid, R.; Mathias, L.; Stefanie, R.; Muhammad, S.; Alexander, V.; Christine, F.; Peter, L. Eur. J. Org. Chem. 2010, 3732.
(g) Ibrar, H.; Mirza, A. Y.; Matthias, L.; Thomas, P.; Christine, F.; Helmar, G.; Peter, L. Eur. J. Org. Chem. 2008, 503.
(h) Satya, P. S.; Rakesh, T.; Akhilesh, K. V. Tetrahedron 2012, 68, 9035.
(i) So, W. Y.; Byung, S. K.; Arun, R. J. J. Am. Chem. Soc. 2012, 134, 11308.
(j) Hao, X.; Shang, f. L.; Hong, X. L.; Hua, F.; Yu, Y. J. Chem. Commun. 2010, 7617.
(k) Patricia, G.; Manuel, A.; Fernndez, R.; Enrique, A. Angew. Chem., Int. Ed. 2009, 48, 5534.
[5] (a) Liu, Y. C.; Huang, Z. Y.; Chen, Q.; Yang, G. F. Tetrahedron 2013, 69, 9025.
(b) Qu, R. Y.; Liu, Y. C.; Wu, Q. Y.; Chen, Q.; Yang, G. F. Tetrahedron 2015, 71, 8123.
[6] (a) Leadbeater, N. E. Chem. Commun. 2005, 2881.
(b) Lussier, T; Herve, G; Enderlin, G; Len, C. RSC Adv. 2014, 4, 462183.
(c) Kappe, C. O. Angew. Chem., Int. Ed. 2004, 43, 6250.
(d) Zhou, Z. Z.; Zhao, P. L.; Huang, W.; Yang, G. F. Adv. Synth. Catal. 2006, 348, 63.
(e) Zhou, Z. Z.; Ji, F. Q.; Cao, M.; Yang, G. F. Adv. Synth. Catal. 2006, 348, 1826.
(f) Liu, Y. C.; Ye, C. J.; Chen, Q.; Yang, G. F. Tetrahedron Lett. 2013, 54, 949.
(g) Liu, Y. C.; Qu, R. Y.; Chen, Q.; Yang, G. F. Tetrahedron 2014, 70, 2746.
(h) Hua, C.; Wu, Q. Y.; Han, F.; Yang, G. F. Chin. Chem. Lett. 2014, 25, 705.
[7] Mei, T. S.; Giri, R.; Maugel, N.; Yu, J. Q. Angew. Chem., Int. Ed. 2008, 47, 5215.
/
| 〈 |
|
〉 |