

含羟基氮杂环卡宾的咪唑盐前体的合成及其原位产生的卡宾配体在Suzuki-Miyaura和Sonogashira反应中的应用
收稿日期: 2016-10-22
修回日期: 2016-12-06
网络出版日期: 2017-02-08
基金资助
山西省基础研究计划项目(No.2015011029)、山西师范大学大学生创新实验项目(No.SD2014CXXM-36)及山西省研究生教育创新(No.2015BY38)资助项目.
Synthesis of Imidazolium Precursors for the Hydroxyl-Group-Modified N-Heterocyclic Carbenes and Applications of the in situ Generated Carbene Ligands in Suzuki-Miyaura and Sonogashira Coupling Reactions
Received date: 2016-10-22
Revised date: 2016-12-06
Online published: 2017-02-08
Supported by
Project supported by the Basic Research Project of Shanxi Province of China (No. 2015011029), the Undergraduate Innovative Experiment Program of Shanxi Normal University (No. SD2014CXXM-36) and the Shanxi Province Education Innovation Project for Postgraduate (No. 2015BY38).
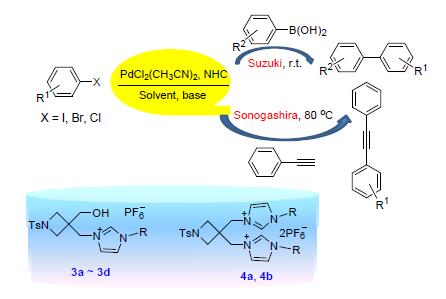
合成了四种含羟基官能团的四元杂环氮杂环卡宾前体咪唑盐,并用核磁共振、红外和单晶衍射等手段对化合物的结构进行了表征.这些咪唑盐前体与钯源PdCl2(CH3CN)2组成的催化剂在碱(KOH)存在下可高效地催化Suzuki-Miyaura偶联反应,该反应在叔丁醇/水(V:V=1:1)的混合溶剂中室温下即可进行,具有条件温和、收率高、绿色环保等优点.此外,PdCl2(CH3CN)2/咪唑盐/CuI的混合催化剂体系即便在小装载量时对构建芳基炔类化合物的Sonogashira偶联反应也表现出良好的催化活性.其中,PdCl2/咪唑盐3b催化剂对这两类偶联反应均表现出了高催化活性.
关键词: 羟基官能团; 氮杂环卡宾; 钯; Suzuki偶联反应; Sonogashira偶联反应
白亚丽 , 李晓维 , 肖雪冬 , 刘佳琦 , 杨俊娟 , 王君文 . 含羟基氮杂环卡宾的咪唑盐前体的合成及其原位产生的卡宾配体在Suzuki-Miyaura和Sonogashira反应中的应用[J]. 有机化学, 2017 , 37(5) : 1258 -1265 . DOI: 10.6023/cjoc201610039
Four imidazolium precursors for the N-heterocyclic carbenes (NHCs) with a hydroxyl functionalized four-mem-bered heterocyclic side arm were synthesized and characterized by IR, XRD and NMR spectroscopies. The corresponding NHC ligands thus generated in situ from these imidazolium precursors in the presence of base such as KOH, together with PdCl2(CH3CN)2, exhibited excellent catalytic activity in Suzuki-Miyaura cross-coupling reactions for the synthesis of a range of biaryl compounds. The reactions could be conducted in the mixed solvent of tert-butyl alcohol/water (V:V=1:1) at room temperature with the advantages of mild conditions, high efficiency as well as environmental friendliness. In addition, although in minor amount, the mixed catalyst system containing imidazolium salts, PdCl2(CH3CN)2 (0.1%) and CuI (1%) exhibited excellent catalytic activity in Sonogashira coupling reaction. In particular, the combination system of PdCl2/imidazolium salt 3b has been shown to be higher catalytically active for both coupling reactions.

[1] Arduengo, A. J.; Harlow, R. L.; Kline, M. J. Am. Chem. Soc. 1991, 113, 361.
[2] Qu, M. N.; He, J. M. Chin. J. Org. Chem. 2011, 31, 1388 (in Chinese). (屈孟男, 何金梅, 有机化学, 2011, 31, 1388.)
[3] Tang, Y.; Yang, F. F.; Nie, S. P.; Wang, L.; Luo, Z. B.; Lu, H. F. Chin. J. Org. Chem. 2015, 35, 705 (in Chinese). (唐演, 杨飞飞, 聂士鹏, 王林, 罗治斌, 陆鸿飞, 有机化学, 2015, 35, 705.)
[4] Zhang, D.; Zi, G. F. Chem. Soc. Rev. 2015, 44, 1898.
[5] Barroso, S.; de Aguiar, S. R. M. M.; Munhá, R. F.; Martins, A. M. J. Organomet. Chem. 2014, 760, 60.
[6] Zhao, N.; Hou, G. H.; Deng, X. B.; Zi, G. F.; Walter, M. D. Dalton Trans. 2014, 43, 8261.
[7] Hameury, S.; de Frémont, P.; Breuil, P. A. R.; Olivier-Bourbigou, H.; Braunstein, P. Inorg. Chem., 2014, 53, 5189.
[8] Ohmiya, H.; Zhang, H.; Shibata, S.; Harada, A.; Sawamura, M. Angew. Chem. 2016, 128, 4855.
[9] (a)Wang, N. X. Chin. J. Org. Chem. 2011, 31, 1319 (in Chinese). (王乃兴, 有机化学, 2011, 31, 1319.) (b)Maluenda, I.; Navarro, O. Molecules 2015, 20, 7528.
[10] Fortman, G. C.; Nolan, S. P. Chem. Soc. Rev. 2011, 40, 5151.
[11] Levin, E.; Ivry, E.; Diesendruck, C. E.; Lemcoff, N. G. Chem. Rev. 2015, 115, 4607.
[12] Schmid, T. E.; Jones, D. C.; Songis, O.; Diebolt, O.; Furst, M. R. L.; Slawin, A. M. Z.; Cazin, C. S. J. Dalton Trans. 2013, 42, 7345.
[13] Neumann, K. T.; Laursen, S. R.; Lindhardt, A. T.; Bang-Andersen, B.; Skrydstrup, T. Org. Lett. 2014, 16, 2216.
[14] Caddick, S.; Cloke, F. G. N.; Clentsmith, G. K. B.; Hitchcock, P. B.; McKerrecher, D.; Titcomb, L. R.; Williams, M. R. V. J. Organomet. Chem. 2001, 617, 635.
[15] Burkhard, J. A.; Wagner, B.; Fischer, H.; Schuler, F.; Müller, K.; Carreira, E. M. Angew. Chem., Int. Ed. 2010, 49, 3524.
[16] Burkhard, J. A.; Guérot, C.; Knust, H.; Rogers-Evans, M.; Carreira, E. M. Org. Lett. 2010, 12, 1944.
[17] Burkhard, J.; Carreira, E. M. Org. Lett. 2008, 10, 3525.
[18] Wuitschik, G.; Rogers-Evans, M.; Buckl, A.; Bernasconi, M.; Märki, M.; Godel, T.; Fischer, H.; Wagner, B.; Parrilla, I.; Schuler, F.; Schneider, J.; Alker, A.; Schweizer, W. B.; Müller, K.; Carreira, E. M. Angew. Chem. 2008, 120, 4588.
[19] Wang, J. W.; Li, Q. S.; Xu, F. B.; Song, H. B.; Zhang, Z. Z. Eur. J. Org. Chem. 2006, 1310.
[20] Yu, L.; Han, Z. Mater. Lett. 2016, 184, 312.
[21] Al-Amin, M.; Akimoto, M.; Tameno, T.; Ohki, Y.; Takahashi, N.; Hoshiya, N.; Shuto, S.; Arisawa, M. Green Chem. 2013, 15, 1142.
[22] Ma, H. C.; Cao, W.; Bao, Z. K.; Lei, Z. Q. Catal. Sci. Technol. 2012, 2, 2291.
[23] Zhao, X. H.; Zhao, Y. Y.; Zhang, J.; Li, X. Appl. Organomet. Chem. 2015, 29, 840.
[24] Yadav, R. R.; Vishwakarma, R. A.; Bharate, S. B. Tetrahedron Lett. 2012, 53, 5958.
[25] Hassine, A.; Sebti, S.; Solhy, A.; Zahouily, M.; Len, C.; Hedhili, M. N.; Fihri, A. Appl. Catal., A 2013, 450, 13.
[26] Siamaki, A. R.; Lin, Y.; Woodberry, K.; Connell, J. W.; Gupton, B. F. J. Mater. Chem. A 2013, 1, 12909.
[27] Iranpoor, N.; Firouzabadi, H.; Motevalli, S.; Rajabi, K. Aust. J. Chem. 2015, 68, 926.
[28] Zhang, G. P.; Li, P. H. Chin. J. Org. Chem. 2010, 30, 117 (in Chinese). (张国平, 李品华, 有机化学, 2010, 30, 117.)
[29] Elumalai, V.; Bjørsvik, H. R. Tetrahedron Lett. 2016, 57, 1224.
[30] Wang, X.; Wang, Z. H.; Wu, Y.; Luo, Y. L.; Zhang, G. F.; Jian, Y. J.; Sun, H. M.; Zhang, W. Q.; Gao, Z. W. Appl. Organomet. Chem. 2016, 30, 831.
[31] Sagadevan, A.; Hwang, K. C. Adv. Synth. Catal. 2012, 354, 3421.
/
| 〈 |
|
〉 |