

1,2-双十六烷基-3-甘油-磷酸乙醇胺的合成研究
收稿日期: 2016-12-09
修回日期: 2017-02-13
网络出版日期: 2017-02-17
基金资助
国家自然科学基金委员会-山东省人民政府海洋科学研究中心联合(No.U1406402)、山东省泰山学者项目、海洋公益性行业科研专项(No.201405038)资助项目.
Synthesis of 1,2-Dihexadecyl-sn-glycero-3-phosphoethanolamine
Received date: 2016-12-09
Revised date: 2017-02-13
Online published: 2017-02-17
Supported by
Project supported by the National Natural Science Foundation of China-Shandong Joint Fund for Marine Science Research Centers (No. U1406402), the Taishan Scholar Project Special Funds, and the Special Fund for Marine Scientific Research in the Public Interest (No. 201405038).
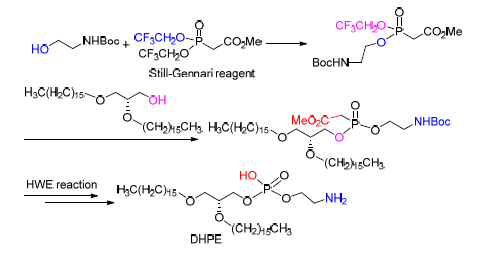
1,2-双十六烷基-3-甘油-磷酸乙醇胺(DHPE)属于甘油磷脂的一种,在生物学方面具有广泛的应用.报道了化合物DHPE的合成,合成路线以(S)-(+)-甘油醇缩丙酮、十六醇、乙醇胺为起始原料,得到了关键中间体1,2-双十六烷基甘油醇和Boc保护乙醇胺,然后通过Still-Gennari试剂将二者进行偶联,随后利用Horner-Wadsworth-Emmons反应高效的脱去磷脂上的乙酸甲酯基团,最终经过8步反应,以32%的总收率得到目标化合物DHPE.
关键词: DHPE; 甘油磷脂; 合成; Horner-Wadsworth-Emmons反应
张越 , 张鹏涛 , 于广利 , 李春霞 . 1,2-双十六烷基-3-甘油-磷酸乙醇胺的合成研究[J]. 有机化学, 2017 , 37(6) : 1456 -1462 . DOI: 10.6023/cjoc201612034
1,2-Dihexadecyl-sn-glycero-3-phosphoethanolamine (DHPE) is one type of glycerophospholipids, and it has a wide range of application in biology. A new and efficient method was developed to synthesis DHPE using (S)-2,2-dimethyl-1,3-dioxolane-4-methanol, hexadecanol and ethanolamine as starting materials. 1,2-Dihexadecyl-sn-glycerol and N-Boc-etha-nolamine were synthesized as two key intermediates, which were coupled under condition of Still-Gennari reagent. Then Horner-Wadsworth-Emmons reaction condition was applied to deprotect methyl acetate group of glycerophospholipid efficiently. The target compound DHPE was obtained in 32% overall yield by 8 steps.

Key words: DHPE; glycerophospholipids; synthesis; Horner-Wadsworth-Emmons reactions
[1] Han, L.; Li, Z. M.; Guo, W. M. Chem Bull. 2005, 68, 408 (in Chinese). (韩亮, 李正名, 郭维明, 化学通报, 2005, 68, 408.)
[2] Caputto, B. L.; Guido, M. E. Biochim. Biophys. Acta 2002, 1583, 1.
[3] Navab, M. J. Lipid. R. 2004, 45, 993.
[4] Snyder, F. Biochem. Lipids. Membr. 1991, 20, 271.
[5] Diagne, A.; Fauvel, J.; Record, M.; Chap, H.; Douste-Blazy, L. Biochim. Biophys. Acta 1984, 793, 221.
[6] Mueller, H. W.; O' Flaherty, J. T.; Wykle, R. L. Lipids 1982, 17, 72.
[7] Record, M.; Tamer, A.; Chap, H.; Douste-Blazy, L. Biochim. Biophys. Acta 1984, 778, 449.
[8] Fukui, S.; Feizi, T.; Galustian, C.; Lawson, A. M.; Chai, W. Nat. Biotechnol. 2002, 20, 1011.
[9] Stoll, M. S.; Feizi, T.; Loveless, R. W.; Chai, W.; Lawson, A. M.; Yuen, C. T. Eur. J. Biochem. 2000, 267, 1795.
[10] Liu, Y.; Feizi, T.; Campanero-Rhodes, M. A.; Childs, R. A.; Zhang, Y. Chem. Biol. 2007, 14, 847.
[11] Abdelmageed, O. H.; Duclos, R. I.; Griffin, R, G.; Siminovitch, D. J.; Ruocco, M. J.; Makriyannis, A. Chem. Phys. Lipids 1989, 50, 163.
[12] Alcaraz, M.; Peng, L.; Klotz, P.; Goeldner, M. J. Org. Chem. 1996, 61, 192.
[13] Song, Y.; Yuan, W.; Luo, Y.; Lu, W. Chin. Chem. Lett. 2012, 23, 154.
[14] Sano, S.; Sumiyoshi, H.; Handa, A.; Tokizane, R.; Nakao, M. Tetrahedron Lett. 2015, 56, 4686.
[15] Bisceglia, A. R.; Orelli, L. Curr. Org. Chem. 2012, 16, 2206.
[16] Al Jasem, Y.; El-Esawi, R.; Thiemann, T. J. Chem. Res. 2014, 38, 453.
[17] Messik, F.; Oberthür, M. Synthesis. 2013, 45, 167.
[18] Sano, S.; Kujime, E.; Takemoto, Y.; Shiro, M.; Nagao, Y. Chem. Pharm. Bull. 2005, 53, 131.
[19] Sano, S.; Takemoto, Y.; Nagao, Y. ARKIVOC 2003, 8, 93.
[20] Casati, S.; Ciuffreda, P.; Santaniello, E. Tetrahedron 2011, 22, 658.
[21] Wawzonek, S.; Klimstra, P. D.; Kallio, R. E. J. Org. Chem. 1960, 25, 621.
/
| 〈 |
|
〉 |