

CeCl3·7H2O催化Groebke-Blackburn-Bienayme反应简便合成咪唑稠杂环化合物(英文)
收稿日期: 2017-01-10
修回日期: 2017-02-12
网络出版日期: 2017-02-20
基金资助
国家自然科学基金(No.30873140)、北京市优秀人才培养(No.20071D0501600227)、北京市教委科技发展(No.KM201010028011)资助项目.
Convenient Synthesis of Imidazo-Fused Heterocycles via CeCl3·7H2O Catalyzed Groebke-Blackburn-Bienayme Reaction
Received date: 2017-01-10
Revised date: 2017-02-12
Online published: 2017-02-20
Supported by
Project supported by the National Natural Science Foundation of China (No. 30873140), the Program for Excellent Talents of Beijing City (No. 20071D0501600227) and the Beijing Municipal Commission of Education (No. KM201010028011).
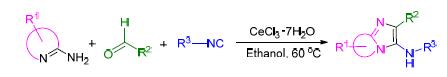
发展了由CeCl3·7H2O催化“一锅法”Groebke-Blackburn-Bienayme(GBB)反应合成咪唑稠杂环化合物的一种简单、高效和环境友好的合成方法.该反应以醛、氨基吖嗪、异腈三组分为原料,CeCl3·7H2O为催化剂,乙醇作为反应介质、60℃反应温度下可高收率合成相应咪唑稠杂环化合物.该方法具有底物范围广,反应时间短,纯化方法简单的优势.通过CeCl3·7H2O和高效催化剂LaCl3·7H2O的对比研究发现,CeCl3·7H2O具有同样高的催化效率,为咪唑稠杂环化合物的合成提供了一种新的便利方法.
张钊瑞 , 徐良 , 唐汉秦 , 吴伯鑫 , 冯迪 , 郭长彬 . CeCl3·7H2O催化Groebke-Blackburn-Bienayme反应简便合成咪唑稠杂环化合物(英文)[J]. 有机化学, 2017 , 37(5) : 1252 -1257 . DOI: 10.6023/cjoc201701024
A simple, efficient and eco-friendly “one-pot” method for the convenient synthesis of imidazo-fused heterocycles has been developed. The reaction was catalyzed by CeCl3·7H2O in ethanol under 60 ℃ via Groebke-Blackburn-Bienayme (GBB) reaction of aldehydes, aminoazines and isocyanides. This method has many advantages of a wide range of substrates, short reaction time and easy purification. In addition, we conducted a comparative study of LaCl3·7H2O with CeCl3·7H2O on their catalytic effect under the same reaction conditions, and found that CeCl3·7H2O was comparable to LaCl3·7H2O as catalyst in GBB reaction.

[1] (a) Ma, Z.; Xiang, Z.; Luo, T.; Lu, K.; Xu, Z.; Chen, J.; Yang, Z. J. Comb. Chem. 2006, 8, 696.
(b) Riva, R.; Banfi, L.; Basso, A.; Cerulli, V.; Guanti, G.; Pani, M. J. Org. Chem. 2010, 15, 5134.
(c) Erb, W.; Neuville, L.; Zhu, J. J. Org. Chem. 2009, 74, 3109.
(d) Zhang, Z. R.; Zheng, X. L.; Guo, C. B. Chin. J. Org. Chem. 2016, 36, 1241 (in Chinese). (张钊瑞, 郑晓霖, 郭长彬, 有机化学, 2016, 36, 1241.)
(e) Singh, S.; Samanta, S. Chin. J. Chem. 2015, 33, 1244.
(f) Wang, H.; Xu, B. Chin. J. Org. Chem. 2015, 35, 588 (in Chinese). (王浩, 许斌, 有机化学, 2015, 35, 588.)
(g) Sun, X. Y.; Wang, W. M.; Ma, J.; Yu, S. Y. Acta Chim. Sinica 2017, 75, 115 (in Chinese). (孙晓阳, 王文敏, 马晶, 俞寿云, 化学学报, 2017, 75, 115.)
(h) Ren, S. B.; Zhang, H. F.; Zhang, J.; Zhang, W.; Liu, Y. K. Chin. J. Org. Chem. 2016, 36, 1954 (in Chinese). (任少波, 张海峰, 张剑, 张巍, 刘运奎, 有机化学, 2016, 36, 1954.)
(i) Xu, W. S.; Zhao, S. J.; Luo, X. P.; Song, J. N.; Liu, J. Q.; Bi, X. H.; Liao, P. Q. Chin. J. Org. Chem. 2015, 35, 2095 (in Chinese). (徐文帅, 赵寿经, 骆晓沛, 宋金娜, 刘建全, 毕锡和, 廖沛球, 有机化学, 2015, 35, 2095.)
(j) Li, Z. H.; Yan, N. N.; Xie, J. W.; Liu, P.; Zhang, J.; Dai, B. Chin. J. Chem. 2015, 33, 589.
[2] Devi, N.; Rawal, R. K.; Singh, V. Tetrahedron 2015, 71, 183.
[3] Berson, A.; Descatoire, V.; Sutton, A.; Fau, D.; Maulny, B.; Vadrot, N.; Feldmann, G.; Berthon, B.; Tordjmann, T.; Pessayre, D. J. Pharmacol. Exp. Ther. 2001, 299, 793.
[4] Harrison, T. S.; Keating, G. M. CNS Drugs 2005, 19, 65.
[5] Kaminski, J. J.; Wallmark, B.; Briving, C.; Andersson, B. M. J. Med. Chem. 1991, 34, 533.
[6] Mizushige, K.; Ueda, T.; Yukiiri, K.; Suzuki, H. Cardiovasc. Drug Rev. 2002, 20, 163.
[7] (a) Palmer, A. M.; Chrismann, S.; Munch, G.; Brehm, C.; Zimmermann, P. J.; Buhr, W.; Senn-Bilfinger, J.; Feth, M. P.; Simon, W. A. Bioorg. Med. Chem. 2009, 17, 368.
(b) Mori, H.; Tanaka, M.; Kayasuga, R.; Masuda, T.; Ochi, Y.; Yamada, H.; Kishikawa, K.; Ito, M.; Nakamura, T. Bone 2008, 43, 840.
[8] Sharma, A.; Li, H. Y. Synlett 2011, 1407.
[9] Huang, Y.; Hu, X. Q.; Shen, D. P.; Chen, Y. F.; Xu, P. F. Mol. Diversity 2007, 11, 73.
[10] Groebke, K.; Weber, L.; Mehlin, F. Synlett 1998, 661.
[11] Che, C.; Xiang, J.; Wang, G. X.; Fathi, R.; Quan, J. M.; Yang, Z. J. Comb. Chem. 2007, 9, 982.
[12] Mert-Balci, F.; Conrad, J.; Meindl, K.; Schulz, T.; Stalke, D.; Beifuss, U. Synthesis 2008, 3649.
[13] McKeown, M. R.; Shaw, D. L.; Fu, H.; Liu, S.; Xu, X.; Marineau, J. J.; Huang, Y.; Zhang, X.; Buckley, D. L.; Kadam, A.; Zhang, Z.; Blacklow, S. C.; Qi, J.; Zhang, W.; Bradner, J. E. J. Med. Chem. 2014, 57, 9019.
[14] Shaabani, A.; Soleimani, E.; Sarvary, A.; Rezayan, H.; Maleki, A. Chin. J. Chem. 2009, 27, 369.
[15] Xi, G. L.; Liu, Z. Q. Tetrahedron 2015, 71, 9602.
[16] Shinde, A. H.; Srilaxmi, M.; Satpathi, B.; Sharada, D. S. Tetrahedron Lett. 2014, 55, 5915.
[17] Odell, L. R.; Nilsson, M.; Gising, J.; Lagerlund, O.; Muthas, D.; Nordqvist, A.; Karlen, A.; Larhed, M. Bioorg. Med. Chem. Lett. 2009, 19, 4790.
[18] Guchhait, S. K.; Madaan, C. Tetrahedron Lett. 2011, 52, 56.
[19] Guchhait, S. K.; Maadan, C. Synlett 2009, 628.
[20] Rousseau, A. L.; Matlaba, P.; Parkinson, C. J. Tetrahedron Lett. 2007, 48, 4079.
[21] Rostamnia, S.; Hassankhanib, A. RSC Adv. 2013, 3, 18626.
[22] Akbarzadeh, R.; Shakibaei, G. I.; Bazgir, A. Monatsh. Chem. 2010, 141, 1077.
[23] Sanaeishoara, T.; Tavakkolia, H.; Mohave, F. Appl. Catal., A 2014, 470, 56.
[24] Shaabani, A.; Soleimani, E.; Maleki, A. Tetrahedron Lett. 2006, 47, 3031.
[25] Lee, C. H.; Hsu, W. S.; Chen, C. H.; Sun, C. M. Eur. J. Org. Chem. 2013, 11, 2201.
[26] Neochoritis, C. G.; Stotani, S.; Mishra, B.; Domling, A. Org. Lett. 2015, 17, 2002.
[27] Kolo, K.; Sajadi, S. M. Lett. Org. Chem. 2013, 10, 688.
[28] Bartoli, G.; Marcantoni, E.; Marcolini, M.; Sambri, L. Chem. Rev. 2010, 110, 6104.
/
| 〈 |
|
〉 |