

铜催化一锅法制备N,N-二甲基苯甲酰胺和苯腈
收稿日期: 2016-12-12
修回日期: 2017-01-20
网络出版日期: 2017-02-20
基金资助
国家自然科学基金(Nos.21472128,J1310008)资助项目.
Synthesis of N,N-Dimethyl Benzamide and Benzonitriles through Copper-Catalyzed One-Pot Reaction
Received date: 2016-12-12
Revised date: 2017-01-20
Online published: 2017-02-20
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21472128, J1310008).
张伟 , 胡晨旭 , 周向葛 . 铜催化一锅法制备N,N-二甲基苯甲酰胺和苯腈[J]. 有机化学, 2017 , 37(5) : 1246 -1251 . DOI: 10.6023/cjoc201612040
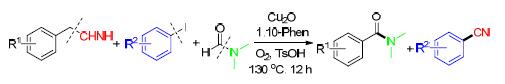
Amide is one of the most important functional groups in nature. Besides the classical synthetic method by using activated acid with amine, several other transition metal-catalyzed protocols have been developed. Aryl nitriles have also attracted substantial attentions as herbicides, natural products, etc. Traditional methods towards aryl nitriles include Sandmeyer and Rosenmund von Braun reaction. In addition of these methods, researchers have explored various kinds of toxic “CN” sources. In continuation of our previous work on copper catalyzed C-CN bond cleavage and C-N formation reactions, herein our recent work of combination of copper-catalyzed amidation of benzyl cyanide and cyanation of aryl iodides by using N,N-dimethyl formamide (DMF) as amide source is reported. A representative procedure for this reaction is as following: benzyl cyanide (1 mmol), iodobenzene (1 mmol), DMF (2 mL), TsOH (1 mmol), Cu2O (0.2 mmol), 1,10-phenanthroline (0.4 mmol) were added into a 10 mL of Schlenk tube. The mixture was stirred at 130 ℃ under O2 atmosphere for 12 h. The reaction mixture was then cooled down to room temperature, quenched with water, and extracted with ethyl acetate. The organic layer was then dried over anhydrous MgSO4, and the solvent was removed in vacuo. The residue was finally purified by column chromatography on silica gel using petroleum ether-ethyl acetate mixture as eluent. A variety of N,N-dimethyl benzamides and benzonitriles were obtained in yields up to 85% and 75%, respectively.
Key words: copper; catalysis; one pot reaction; amidation; cyanation
[1] (a) Larock, R. C. Comprehensive Organic Transformations, 2nd ed., Wiley-VCH, Weinheim, 1999.
(b) Sewald, N.; Jakubke, H. D. Peptides: Chemistry and Biology. Wiley-VCH, Weinheim, 2002.
(c) Bray, B. L. Nat. Rev. Drug Discovery 2003, 2, 587.
(d) Cupido, T.; Tulla-Puche, J.; Spengler, J.; Albericio, F. Curr. Opin. Drug Discovery Dev. 2007, 10, 768.
(e) Wang, W. L.; Luo, H.; Gao, L. X.; Sheng, L.; Zhou, Y. B.; Li, J.; Li, J. Y.; Feng, B. N. Chin. J. Org. Chem. 2016, 36, 2142 (in Chinese). (王文龙, 骆欢, 高雅, 高立信, 盛丽, 周宇波, 李佳, 李静雅, 冯柏年, 有机化学, 2016, 36, 2142.)
(f) Ye, S.; Li, H.; Yang, W.; Luo, Y. J. Am. Chem. Soc. 2014, 136, 1206.
(g) Huang, J.; Tian, K.; Luo, Y. J. Phys. Chem. C 2016, 120, 15322.
(h) Pelay-Gimeno, M.; Glas, A.; Koch, O.; Grossmann, T. N. Angew. Chem., Int. Ed. 2015, 54, 8896.
[2] (a) Humphrey, J. M.; Chamberlin, A. R. Chem. Rev. 1997, 97, 2243.
(b) Li, Y.; Li, B. J.; Yang, X. L.; Shi, Y. X.; Miao, H. J.; Ling, Y. Chin. J. Org. Chem. 2011, 31, 1411 (in Chinese). (李映, 李宝聚, 杨新玲, 石延霞, 苗宏健, 凌云, 有机化学, 2011, 31, 1411.)
(c) Qian, P. F.; Wan, H. X.; Jiang, J.; Hu, Y. W.; Chen, X. B.; Zhang, S. L. Chin. J. Org. Chem. 2016, 36, 1878 (in Chinese). (钱彭飞, 万惠新, 蒋静, 胡延维, 陈晓蓓, 张士磊, 有机化学, 2016, 36, 1878.)
(d) Bertolacci, L.; Romeo, E.; Piomelli, D.; Garau, G. J. Am. Chem. Soc. 2013, 135, 22.
(e) Zhang, D. Q.; Xu, G. F.; Liu, Y. H.; Wang, D. Q.; Yan, X. L.; Yuan, D. K. Chin. J. Org. Chem. 2015, 35, 2191 (in Chinese). (张大强, 徐高飞, 刘艳红, 王道全, 杨新玲, 袁德凯, 有机化学, 2015, 35, 2191.)
[3] Schoenberg, A.; Bartoletti, I.; Heck, R. F. J. Org. Chem. 1974, 39, 3318.
[4] For some of selected examples, see: (a) Wu, X. F.; Neumann, H.; Beller, M. Chem.-Asian J. 2010, 5, 2168.
(b) Tambade, P. J.; Patil, Y. P.; Bhanage, B. M. Appl. Organomet. Chem. 2009, 23, 235.
(c) Brennfuhrer, A.; Neumann, H.; Beller, M. Angew. Chem., Int. Ed. 2009, 48, 4114.
(d) Martinelli, J. R.; Watson, D. A.; Freckmann, D. M. M.; Barder, T. E.; Buchwald, S. L. J. Org. Chem. 2008, 73, 7102.
(e) Bjerglund, K.; Lindhardt, A. T.; Skrydstrup, T. J. Org. Chem. 2012, 77, 3793.
(f) Andersen, T. L.; Frederiksen, M. W.; Domino, K.; Skrydstrup, T. Angew. Chem., Int. Ed. 2016, 55, 10396.
(g) Hao, W.; Liu, H.; Yin, L.; Cai, M. J. Org. Chem. 2016, 81, 4244.
[5] (a) Ren, W.; Yamane, M. J. Org. Chem. 2009, 74, 8332.
(b) Wieckowska, A.; Fransson, R.; Odell, L. R.; Larhed, M. J. Org. Chem. 2011, 76, 978.
(c) Dumbris, S. M.; McElwee-White, L. J. Org. Chem. 2009, 74, 8862.
[6] (a) Wieckowska, A.; Fransson, R.; Odell, L. R.; Larhed, M. J. Org. Chem. 2011, 76, 978.
(b) Ren, W.; Yamane, M. J. Org. Chem., 2010, 75, 8410.
(c) Roberts, B.; Liptrot, D.; Alcaraz, L.; Luker, T.; Stocks, M. J. Org. Lett. 2010, 12, 4280.
(d) Ren, E.; Yamane, M. J. Org. Chem. 2010, 75, 3017.
[7] Corey, E. J.; Hegedus, L. S. J. Am. Chem. Soc. 1969, 91, 1233.
[8] (a) Cunico, R. F.; Pandey, R. K. J. Org. Chem. 2005, 70, 9048.
(b) Cunico, R. F.; Maity, B. C. Org. Lett. 2003, 5, 4947.
(c) Cunico, R. F.; Maity, B. C. Org. Lett. 2002, 4, 4357.
[9] Lindsay, C. M.; Widdowson, D. A. J. Chem. Soc., Perkin Trans. 1 1988, 569.
[10] Wan, Y.; Alterman, M.; Larhed, M.; Hallberg, A. J. Comb. Chem. 2003, 5, 82.
[11] (a) Jo, Y.; Ju, J.; Choe, J.; Song, K. H.; Lee, S. J. Org. Chem. 2009, 74, 6358.
(b) Ju, J.; Jeong, M.; Moon, J.; Jung, H. M.; Lee, S. Org. Lett. 2007, 9, 4615.
(c) Hosoi, K.; Nozaki, K.; Hiyama, T. Org. Lett. 2002, 4, 2849.
(d) Chen, J.; Feng, J-B.; Natte, K.; Wu, X. Chem. Eur. J. 2015, 21, 16370.
(e) Wu, X.; Zhao, Y.; Ge, H. J. Am. Chem. Soc. 2015, 137, 4924.
[12] (a) Kleemann, A.; Engel, J.; Kutscher, B.; Reichert, D. Pharmaceutical Substances: Syntheses, Patents, Applications, 4th ed., Georg Thieme Verlag, Stuttgart, 2001.
(b) Ao, Y. F.; Wang, Q. Q.; Wang, D. X. Chin. J. Org. Chem. 2016, 36, 2333 (in Chinese). (敖宇飞, 王其强, 王德先, 有机化学, 2016, 36, 2333.)
[13] (a) Sandmeyer, T. Ber. Dtsch. Chem. Ges. 1884, 17, 2650.
(b) Rosenmund, K. W.; Struck, E. Ber. Dtsch. Chem. Ges. 1919, 52, 1749.
[14] (a) Zanon, J.; Klapars, A.; Buchwald, S. L. J. Am. Chem. Soc. 2003, 125, 2890.
(b) Yang, C.; Williams, J. M. Org. Lett. 2004, 6, 2837.
(c) Buono, F. G.; Chidambaram, R.; Mueller, R. H.; Waltermire, R. E. Org. Lett. 2008, 10, 5325.
(d) Jia, X.; Yang, D.; Zhang, S.; Cheng, J. Org. Lett. 2009, 11, 4716.
(e) Mondal, B.; Acharyya, K.; Howlader, P.; Sarathi Mukherjee, P. J. Am. Chem. Soc. 2016, 138, 1709.
(f) Yang, C.; Hu, S.; Wang, X. Org. Biomol. Chem. 2015, 13, 2541.
[15] Chen, X.; Hao, X. S.; Goodhue, C. E.; Yu, J.-Q. J. Am. Chem. Soc. 2006, 128, 6790.
[16] Ding, S. T.; Jiao, N. J. Am. Chem. Soc. 2011, 133, 12374.
[17] Luo, F.; Chu, C.; Cheng, C. Organometallics 1998, 17, 1025
[18] (a) Sundermeier, M.; Zapf, A.; Beller, M. Angew. Chem., Int. Ed. 2003, 42, 1661.
(b) Schareina, T.; Zapf, A.; Cotte, A.; Gotta, M.; Beller, M. Adv. Synth. Catal. 2011, 353, 777.
(c) Jiang, Z.; Huang, Q.; Chen, S.; Long, L.; Zhou, X. Adv. Synth. Catal. 2012, 354, 589.
(d) Zheng, S.; Yu, C.; Shen, Z. Org. Lett. 2012, 14, 3644.
(e) Wen, Q.; Jin, J.; Mei, Y.; Lu, P.; Wang, Y. Eur. J. Org. Chem. 2013, 4032.
(f) Kim, J.; Kim, H. J.; Chang, S. Angew. Chem., Int. Ed. 2012, 51, 11948.
(g) Malapit, C. A.; Reeves, J. T.; Senanayake, C. H. Angew. Chem., Int. Ed. 2016, 55, 326.
(h) Ping, Y.; Ding, Q.; Peng, Y. ACS Catal. 2016, 6, 5989.
(i) Mishra, A.; Vats, T. K.; Deb, I. J. Org. Chem. 2016, 81, 6525.
[19] Hu, C.; Yan, X.; Zhou, X.; Li, Z. Org. Biomol. Chem. 2013, 11, 8179.
[20] Cristau, H. J.; Ouali, A.; Spindler, J. F.; Taillefer, M. Chem. Eur. J. 2005, 11, 2483.
[21] Lamani, M.; Prabhu, K. R. Angew. Chem., Int. Ed. 2010, 49, 6622.
[22] Suzuki, Y.; Moriyama, K.; Togo, H. Tetrahedron 2011, 67, 7956.
[23] Enthaler, S. Chem. Eur. J. 2011, 17, 9316.
[24] Bai, C. H.; Yao, X. F.; Li Y. W. ACS Catal. 2015, 5, 884.
[25] Motoyama, Y.; Mitsui, K.; Ishida, T.; Nagashima, H. J. Am. Chem. Soc. 2005, 127, 13150.
[26] Hu, L.; Liu, X.; Liao, X. B. Angew. Chem., Int. Ed. 2016, 55, 9743.
[27] Priyadarshini, S.; Joseph, P. J. A.; Kantam, M. L. RSC Adv. 2013, 3, 18283.
[28] Chen, W. F.; Li, K. B.; Hu, Z. Q.; Wang, L. L.; Lai, G. Q.; Li, Z. F. Organometallics 2011, 30, 2026.
[29] Sawant, D. N.; Wagh, Y. S.; Bhatte, K. D.; Bhanage, B. M. J. Org. Chem. 2011, 76, 5489.
[30] Liu, Z. J.; Zhang, J.; Chen, S. L.; Shi, E.; Xu, Y.; Wan, X. B. Angew. Chem., Int. Ed. 2012, 51, 3231.
/
| 〈 |
|
〉 |