

过渡金属催化的偶联反应在合成C-糖苷中的应用
收稿日期: 2016-12-08
修回日期: 2017-02-13
网络出版日期: 2017-02-27
基金资助
国家自然科学基金(No.21202166)和江西省教育厅科学技术研究(No.GJJ150328)资助项目.
Applications of Transition Metal Catalyzed Coupling Reactions in the Synthesis of C-Glycosides
Received date: 2016-12-08
Revised date: 2017-02-13
Online published: 2017-02-27
Supported by
Project supported by the National Natural Science Foundation of China (No. 21202166) and the Science and Technology Project Founded by the Education Department of Jiangxi Province (No. GJJ150328).
廖进喜 , 刘慧 , 孙建松 . 过渡金属催化的偶联反应在合成C-糖苷中的应用[J]. 有机化学, 2017 , 37(6) : 1382 -1391 . DOI: 10.6023/cjoc201612031
C-Glycosides in which the interglycosidic oxygen atom have been replaced by carbon atom are widely found in natural products and drug molecules. They have better enzymatic and hydrolytic stablility compared to their corresponding O-glycosides and N-glycosides. The syntheses of them have received considerable attention because of their unique chemical structure and extensive application value. Rencent advances in the synthesis of C-glycosides through coupling reactions catalyzed by transition metal are summarized in this review. A summary of the advantages and disadavatages of different synthetic methods will be beneficial to the develepment of new synthetic method for C-glycosides.
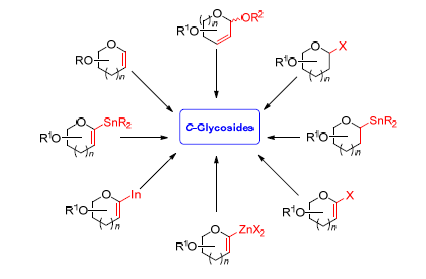
Key words: C-glycosides; synthesis; transition metal catalyzed; coupling reactions
[1] Zou, W. Curr. Top. Med. Chem. 2005, 5, 1363.
[2] Hultin, P. G. Curr. Top. Med. Chem. 2005, 5, 1299.
[3] (a) Cao, X.; Tian, Y.; Zhang, T.; Li, X.; Ito, Y. J. Chromatogr. A 1999, 855, 709.
(b) Nomura, S.; Sakamaki, S.; Hongu, M.; Kawanishi, E.; Koga, Y.; Sakamoto, T.; Yamamoto, Y.; Ueta, K.; Kimata, H.; Nakayama, K.; Tsuda-Tsukimoto, M. J. Med. Chem. 2010, 53, 6355.
[4] Moore, R. E.; Scheuer, P. J. Science 1971, 172, 495.
[5] (a) De Clercq, E. J. Med. Chem. 2016, 59, 2301.
(b) Lalitha, K.; Muthusamy, K.; Prasad, Y. S.; Vemula, P. K.; Nagarajan, S. Carbohydr. Res. 2015, 402, 158.
(c) Yuan, X. J.; Linhardt, R. J. Curr. Top. Med. Chem. 2005, 5, 1393.
(d) Du, Y.; Linhardt, R. J.; Vlahov, I. R. Tetrahedron 1998, 54, 9913.
(e) Lee, D. Y. W.; He, M. S. Curr. Top. Med. Chem. 2005, 5, 1333.
(f) Wellington, K. W.; Benner, S. A. Nucleosides Nucleotides Nucleic Acids 2006, 25, 1309.
(g) Li, X.; Zhu, J. Eur. J. Org. Chem. 2016, 2016, 4724.
[6] (a) Yang, D.-C.; Zhang, Q.-H.; Xiong, W.-W.; Yuan, L.-G.; Cai, Q.-S.; Yang, M.-M.; Li, X.; Jiang, Y.-J.; Liu, Y.; Li, P.; Xu, Z.-S.; Sun, P.-P.; Geng, H.-L. Chin. J. Org. Chem. 2015, 35, 961 (in Chinese).(袁定重, 张庆华, 廖世军, 熊文文, 元利刚, 蔡奇胜, 杨梦梅, 李雄, 蒋烨佳, 刘妍, 李萍, 徐贞帅, 孙盼盼, 耿会玲, 有机化学, 2015, 35, 961.)
(b) Zhang, J.; Lu, Q.-Q.; Liu, C.; Lei, A.-W. Chin. J. Org. Chem. 2015, 35, 743 (in Chinese).(张剑, 陆庆全, 刘超, 雷爱文, 有机化学, 2015, 35, 743.)
(c) Liu, J.; Zhu, Q.-R.; Du, J.-Z.; Xiu, L. Chin. J. Org. Chem. 2015, 35, 15 (in Chinese).(刘杰; 朱庆仁; 杜娟张; 袖丽, 有机化学, 2015, 35, 15.)
(d) Zhang, W.-M.; Dai, J.-J.; Xu, H.-J. Chin. J. Org. Chem. 2015, 35, 1820 (in Chinese).(张文曼, 戴建军, 许华建, 有机化学, 2015, 35, 1820.)
[7] Bergstrom, D. E.; Ruth, J. L. J. Am. Chem. Soc. 1976, 98, 1587.
[8] Arai, I.; Daves, G. D. J. Am. Chem. Soc. 1978, 100, 287.
[9] Daves, G. D. Acc. Chem. Res. 1990, 23, 201.
[10] Cheng, J. C. Y.; Daves, G. D. J. Org. Chem. 1987, 52, 3083.
[11] Ramnauth, J.; Poulin, O.; Rakhit, S.; Maddaford, S. P. Org. Lett. 2001, 3, 2013.
[12] Beletskaya, I. P.; Cheprakov, A. V. Chem. Rev. 2000, 100, 3009.
[13] Xiong, D. C.; Zhang, L. H.; Ye, X. S. Org. Lett. 2009, 11, 1709.
[14] Li, H. H.; Ye, X. S. Org. Biomol. Chem. 2009, 7, 3855.
[15] Kusunuru, A. K.; Jaladanki, C. K.; Tatina, M. B.; Bharatam, P. V.; Mukherjee, D. Org. Lett. 2015, 17, 3742.
[16] Lei, M.; Gao, L.; Yang, J.-S. Tetrahedron Lett. 2009, 50, 5135.
[17] Bai, Y.; Leow, M.; Zeng, J.; Liu, X. W. Org. Lett. 2011, 13, 5648.
[18] Dunkerton, L. V.; Euske, J. M.; Serino, A. J. Carbohydr. Res. 1987, 171, 89.
[19] Brakta, M.; Lhoste, P.; Sinou, D. J. Org. Chem. 1989, 54, 1890.
[20] Friesen, R. W.; Sturino, C. F. J. Org. Chem. 1990, 55, 2572.
[21] Lehmann, U.; Awasthi, S.; Minehan, T. Org. Lett. 2003, 5, 2405.
[22] Tius, M. A.; Gomez-Galeno, J.; Gu, X. Q.; Zaidi, J. H. J. Am. Chem. Soc. 1991, 113, 5775.
[23] Ousmer, M.; Boucard, V.; Lubin-Germain, N.; Uziel, J.; Augé, J. Eur. J. Org. Chem. 2006, 1216.
[24] Koester, D. C.; Leibeling, M.; Neufeld, R.; Werz, D. B. Org. Lett. 2010, 12, 3934.
[25] Schmidt, R. R.; Preuss, R.; Betz, R. Tetrahedron Lett. 1987, 28, 6591.
[26] Koester, D. C.; Kriemen, E.; Werz, D. B. Angew. Chem., Int. Ed. 2013, 52, 2985.
[27] Belosludtsev, Y. Y.; Bhatt, R. K.; Falck, J. R. Tetrahedron Lett. 1995, 36, 5881.
[28] Zhu, F.; Rourke, M. J.; Yang, T. Y.; Rodriguez, J.; Walczak, M. A. J. Am. Chem. Soc. 2016, 138, 12049.
[29] Jeanneret, V.; Meerpoel, L.; Vogel, P. Tetrahedron Lett. 1997, 38, 543.
[30] Potuzak, J. S.; Tan, D. S. Tetrahedron Lett. 2004, 45, 1797.
[31] (a) Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147.
(b) Liu, C.; Zhang, H.; Shi, W.; Lei, A. Chem. Rev. 2011, 111, 1780.
[32] Liu, M.; Niu, Y.; Wu, Y. F.; Ye, X. S. Org. Lett. 2016, 18, 1836.
[33] Myers, A. G.; Tanaka, D.; Mannion, M. R. J. Am. Chem. Soc. 2002, 124, 11250.
[34] Rodriguez, N.; Goossen, L. J. Chem. Soc. Rev. 2011, 40, 5030.
[35] Xiang, S.; Cai, S.; Zeng, J.; Liu, X. W. Org. Lett. 2011, 13, 4608.
[36] Zeng, J.; Ma, J.; Xiang, S.; Cai, S.; Liu, X. W. Angew. Chem., Int. Ed. 2013, 52, 5134.
[37] Kito, K.; Ookura, R.; Yoshida, S.; Namikoshi, M.; Ooi, T.; Kusumi, T. Org. Lett. 2008, 10, 225.
[38] Ma, J.; Xiang, S.; Jiang, H.; Liu, X.-W. Eur. J. Org. Chem. 2015, 2015, 949.
[39] Kusunuru, A. K.; Yousuf, S. K.; Tatina, M.; Mukherjee, D. Eur. J. Org. Chem. 2015, 2015, 459.
[40] Bai, Y.; Kim, L. M. H.; Liao, H.; Liu, X.-W. J. Org. Chem. 2013, 78, 8821.
[41] Trost, B. M.; McEachern, E. J.; Toste, F. D. J. Am. Chem. Soc. 1998, 120, 12702.
[42] Nicolaou, K. C.; Sato, M.; Miller, N. D.; Gunzner, J. L.; Renaud, J.; Untersteller, E. Angew. Chem., Int. Ed. 1996, 35, 889.
[43] Bai, Y.; Leng, W. L.; Li, Y.; Liu, X. W. Chem. Commun. (Camb.) 2014, 50, 13391.
[44] Readman, S. K.; Marsden, S. P.; Hodgson, A. Synlett 2000, 1628.
[45] (a) Adlington, R. M.; Baldwin, J. E.; Basak, A.; Kozyrod, R. P. J. Chem. Soc., Chem. Commun. 1983, 944.
(b) Dunach, E.; Esteves, A. P.; Freitas, A. M.; Medeiros, M. J.; Olivero, S. Tetrahedron Lett. 1999, 40, 8693.
[46] Gong, H. G.; Sinisi, R.; Gagne, M. R. J. Am. Chem. Soc. 2007, 129, 1908.
[47] Gong, H. G.; Gagne, M. R. J. Am. Chem. Soc. 2008, 130, 12177.
[48] Gong, H. G.; Andrews, R. S.; Zuccarello, J. L.; Lee, S. J.; Gagne, M. R. Org. Lett. 2009, 11, 879.
[49] (a) Zhao, C.; Jia, X.; Wang, X.; Gong, H. J. Am. Chem. Soc. 2014, 136, 17645.
(b) Jia, X.; Zhang, X.; Qian, Q.; Gong, H. Chem. Commun. (Camb.) 2015, 51, 10302.
[50] Zheng, M.; Xue, W.; Xue, T.; Gong, H. Org. Lett. 2016, 18, 6152.
[51] Liu, C. F.; Xiong, D. C.; Ye, X. S. J. Org. Chem. 2014, 79, 4676.
[52] Nicolas, L.; Angibaud, P.; Stansfield, I.; Bonnet, P.; Meerpoel, L.; Reymond, S.; Cossy, J. Angew. Chem. 2012, 124, 11263.
[53] Juhász, Z.; Micskei, K.; Gál, E.; Somsák, L. Tetrahedron Lett. 2007, 48, 7351.
[54] Andrews, R. S.; Becker, J. J.; Gagné, M. R. Angew. Chem. 2010, 122, 7432.
[55] Andrews, R. S.; Becker, J. J.; Gagne, M. R. Org. Lett. 2011, 13, 2406.
[56] Andrews, R. S.; Becker, J. J.; Gagne, M. R. Angew. Chem., Int. Ed. 2012, 51, 4140.
[57] Ramnauth, J.; Poulin, O.; Bratovanov, S. S.; Rakhit, S.; Maddaford, S. P. Org. Lett. 2001, 3, 2571.
/
| 〈 |
|
〉 |