

新型含吡啶环结构的吡唑肟酯类衍生物的合成与生物活性研究
收稿日期: 2017-01-20
修回日期: 2017-02-21
网络出版日期: 2017-02-27
基金资助
国家自然科学基金(No.21372135)、江苏省“六大人才高峰”(No.2013-SWYY-013)和南通市科技计划(Nos.CP12013002,MS22015020)资助项目.
Synthesis and Bioactivities of Novel Pyrazole Oxime Ester Derivatives Containing Pyridyl Moiety
Received date: 2017-01-20
Revised date: 2017-02-21
Online published: 2017-02-27
Supported by
Project supported by the National Natural Science Foundation of China (No. 21372135), the Research Foundation of the Six People Peak of Jiangsu Province (No. 2013-SWYY-013), and the Science and Technology Project Fund of Nantong City (Nos. CP12013002, MS22015020).
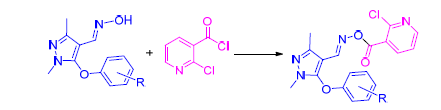
为了寻找具有较好生物活性的吡唑类衍生物,通过活性亚结构拼接的方法,设计制备了一系列未见文献报道的含取代吡啶结构的吡唑肟酯化合物.通过1H NMR、13C NMR和元素分析对目标化合物的结构进行了表征.初步的生物活性测试结果显示,部分化合物表现出一定的杀虫活性.在测试浓度为500 μg/mL时,有7个化合物对粘虫的杀灭活性可达60%~100%,6个化合物对蚜虫的杀死率可达50%~90%.当测试浓度降为100 μg/mL时,1,3-二甲基-5-(4-氯苯氧基)吡唑-4-甲醛-O-(2-氯吡啶-3-甲酰基)肟(5f)和1,3-二甲基-5-(4-甲基苯氧基)吡唑-4-甲醛-O-(2-氯吡啶-3-甲酰基)肟(5j)对蚜虫仍表现出一定的杀虫活性,其杀死率均为50%.1,3-二甲基-5-(3-氟苯氧基)吡唑-4-甲醛-O-(2-氯吡啶-3-甲酰基)肟(5b)和5f在测试浓度为500 μg/mL时对褐飞虱的杀死率均为100%.另外,1,3-二甲基-5-(4-氟苯氧基)吡唑-4-甲醛-O-(2-氯吡啶-3-甲酰基)肟(5c),1,3-二甲基-5-(3-氯苯氧基)吡唑-4-甲醛-O-(2-氯吡啶-3-甲酰基)肟(5e),1,3-二甲基-5-(4-三氟甲氧基苯氧基)吡唑-4-甲醛-O-(2-氯吡啶-3-甲酰基)肟(5i)和5j对人肝癌(HepG2)细胞株显示出明显的抗肿瘤活性,其IC50值分别为2.6,4.6,1.8和1.1 μmol/L.
戴红 , 陈佳 , 洪宇 , 袁斌颖 , 范崇光 , 马瑞媛 , 梁志鹏 , 石健 . 新型含吡啶环结构的吡唑肟酯类衍生物的合成与生物活性研究[J]. 有机化学, 2017 , 37(6) : 1542 -1547 . DOI: 10.6023/cjoc201701042
In order to explore novel pyrazole derivatives with good biological activities, a series of novel pyrazole oxime ester compounds containing pyridyl moiety were designed and synthesized according to the method of active substructure combination. The structures of the target compounds were determined by 1H NMR, 13C NMR and elemental analysis. Preliminary bioassay data indicated that some of the title compounds showed certain insecticidal activities. At a concentration of 500 μg/mL, seven compounds exhibited insecticidal activity against Oriental armyworm with 50%~90%, and six compounds exhibited insecticidal activity against Aphis medicaginis with 50%~90%. When the dosage was lowered to 100 μg/mL, 1,3-dimethyl-5-(4-chlorophenoxy)pyrazole-4-formyl-O-(2-chloropyridin-3-formyl)oxime (5f) and 1,3-dimethyl-5-(4-methylphenoxy)pyra-zole-4-formyl-O-(2-chloropyridin-3-formyl)oxime (5j) were still active against Aphis medicaginis with inhibitory values of 50% and 50%, respectively. Insecticidal activities against Nilaparvata lugens of 1,3-dimethyl-5-(3-fluorophenoxy)pyrazole-4-formyl-O-(2-chloropyridin-3-formyl)oxime (5b) and 5f were both 100% at 500 μg/mL. Additionally, 1,3-dimethyl-5-(4-fluorophenoxy)pyrazole-4-formyl-O-(2-chloropyridin-3-formyl)oxime (5c), 1,3-dimethyl-5-(3-chlorophenoxy)-pyrazole-4-for-myl-O-(2-chloropyridin-3-formyl)oxime (5e), 1,3-dimethyl-5-(4-trifluoromethoxyphenoxy)pyrazole-4-formyl-O-(2-chloro-pyridin-3-formyl)oxime (5i) and 5j displayed good anti-tumor activity against HepG2 cells with IC50 values of 2.6, 4.6, 1.8和1.1 μmol/L, respectively.

Key words: pyridine; pyrazole; synthesis; biological activity
[1] Li, Y.; Zhang, H. Q.; Liu, J.; Yang, X. P.; Liu, Z. J. J. Agric. Food Chem. 2006, 54, 3636.
[2] Dai, H.; Li, Y. Q.; Du, D.; Qin, X.; Zhang, X.; Yu, H. B.; Fang, J. X. J. Agric. Food Chem. 2008, 56, 10805.
[3] Hamaguchi, H.; Kajihara, O.; Katoh, M. J. Pestic. Sci. 1995, 20, 173.
[4] Motoba, K.; Nishizawa, H.; Suzuki, T.; Hamaguchi, H.; Uchida, M.; Funayama, S. Pestic. Biochem. Physiol. 2000, 67, 73.
[5] Park, H. J.; Lee, K.; Park, S. J.; Ahn, B.; Lee, J. C.; Cho, H. Y.; Lee, K. I. Bioorg. Med. Chem. Lett. 2005, 15, 3307.
[6] Hamaguchi, H.; Kajihara, O.; Katoh, M. J. Pestic. Sci. 1995, 20, 173.
[7] Swanson, M. B.; Ivancic, W. A.; Saxena, A. M.; Allton, J. D.; O'Brien, G. K.; Suzuki, T.; Nishizawa, H.; Nokata, M. J. Agric. Food Chem. 1995, 43, 513.
[8] Lahm, G. P., Selby, T. P.; Freudenberger, J. H.; Stevenson, T. M.; Myers, B. J.; Seburyamo, G.; Smith, B. K; Flexner, L.; Clark, C. E.; Cordova, D. Bioorg. Med. Chem. Lett. 2005, 15, 4898.
[9] Penning, T. D.; Talley, J. J.; Bertenshaw, S. R.; Carter, J. S.; Collins, P. W.; Docter, S.; Graneto, M. J.; Lee, L. F.; Malecha, J. W.; Miyashiro, J. M.; Rogers, R. S.; Rogier, D. J.; Yu, S. S.; Anderson, G. D.; Burton, E. G.; Cogburn, J. N.; Gregory, S. A.; Koboldt, C. M.; Perkins, W. E. Seibert, K.; Veenhuizen, A. W.; Zhang, Y. Y.; Isakson, P. C. J. Med. Chem. 1997, 40, 1347.
[10] Teng, M.; Zhu, J. J.; Johnson, M. D.; Chen, P.; Kornmann, J.; Chen, E. T.; Blasina, A.; Register, J.; Anderes, K.; Rogers, C.; Deng, Y. L.; Ninkovic, S.; Grant, S.; Hu, Q. Y.; Lundgren, K.; Peng, Z. W.; Kania, R. S. J. Med. Chem. 2007, 50, 5253.
[11] Ouyang, G. P.; Cai, X. J.; Chen, Z.; Song, B. A.; Bhadury, P. S.; Yang, S.; Jin, L. H.; Xue, W.; Hu, D. Y.; Zeng, S. J. Agric. Food Chem. 2008, 56, 60.
[12] Dai, H.; Shi, L.; Zhang, H. J.; Li, Y. Q.; Fang, J. X.; Shi, Y. J. Chin. J. Org. Chem. 2012, 32, 1060 (in Chinese). (戴红, 施磊, 张海军, 李永强, 方建新, 石玉军, 有机化学, 2012, 32, 1060.)
[13] Wang, X.; Wang, C. Q.; Fu, C. R.; Zou, X. M. Chin. J. Org. Chem. 2015, 35, 92 (in Chinese). (王鑫, 王朝强, 傅翠蓉, 邹小毛, 有机化学, 2015, 35, 92.)
[14] Dai, H.; Zhuang. H. Y.; Shi, L.; Li, G.; Zhang, H. J.; Fang, Y.; Dai, B. J. Chin. J. Org. Chem. 2015, 35, 2399 (in Chinese). (戴红, 庄辉阳, 施磊, 李刚, 张海军, 方源, 戴宝江, 有机化学, 2015, 35, 2399.)
[15] Tian, Z. Z.; Shao, X. S.; Li, Z.; Qian, X. H.; Huang, Q. C. J. Agric. Food Chem. 2007, 55, 2288.
[16] Lu, S. Y.; Shao, X. S.; Li, Z.; Xu, Z. P.; Zhao, S. S.; Wu, Y. L.; Xu, X. Y. J. Agric. Food Chem. 2012, 60, 322.
[17] Selby, T. P.; Lahm, G. P.; Stevenson, T. M.; Hughes, K. A.; Cordova, D.; Annan, I. B.; Barry, J. D.; Benner, E. A.; Currie, M. J.; Pahutski, T. F. Bioorg. Med. Chem. Lett. 2013, 23, 6341.
[18] Song, B. A.; Liu, X. H.; Yang, S.; Hu, D. Y.; Jin, L. H.; Zhang, Y. T. Chin. J. Org. Chem. 2005, 25, 507 (in Chinese). (宋宝安, 刘新华, 杨松, 胡德禹, 金林红, 张玉涛, 有机化学, 2005, 25, 507.)
[19] Ouyang, G. P.; Chen, Z.; Cai, X. J.; Song, B. A.; Bhadury, P. S.; Yang, S.; Jin, L. H.; Xue, W.; Hu, D. Y., Zeng, S. Bioorg. Med. Chem. 2008, 16, 9699.
[20] Shi, Y. J.; Wang, S. L.; He, H. B.; Li, Y.; Li, Y.; Fang, Y.; Dai, H. Chin. J. Org. Chem. 2015, 35, 1785 (in Chinese). (石玉军, 王森林, 何海兵, 李钰, 李阳, 方源, 戴红, 有机化学, 2015, 35, 1785.)
[21] Dai, H.; Li, H.; Jin. Z. C.; Liu, W. Y.; Xiao, Y.; He, H. B.; Wang, Q. M.; Shi, Y. J. Chin. J. Org. Chem. 2016, 36, 185 (in Chinese). (戴红, 李宏, 金智超, 刘文永, 肖瑶, 何海兵, 汪清民, 石玉军, 有机化学, 2016, 36, 185.)
[22] Song, H. J.; Liu, Y. X.; Xiong, L. X.; Li, Y. Q.; Yang, N.; Wang, Q. M. J. Agric. Food Chem. 2013, 61, 8730.
[23] Liu, X. H.; Cui, P.; Song, B. A.; Bhadury, P. S.; Zhu, H. L.; Wang, S. F. Bioorg. Med. Chem. 2008, 16, 4075.
[24] Park, M. S.; Park, H. J.; Park, K. H.; Lee, K. I. Synth. Commun. 2004, 34, 1541.
[25] Ma, J. A.; Huang, R. Q.; Feng, L.; Song, J.; Qiu, D. W. Chem. Res. Chin. Univ. 2003, 19, 297.
[26] Dai, H.; Xiao, Y. S.; Li, Z.; Xu, X. Y.; Qian, X. H. Chin. Chem. Lett. 2014, 25, 1014.
[27] Liu, J. C.; Liu, Y. J.; He, H. W. Chin. J. Org. Chem. 2015, 35, 462 (in Chinese). (刘建超, 刘勇军, 贺红武, 有机化学, 2015, 35, 462.)
[28] Song, B. A.; Yang, S.; Zhong, H. M.; Jin, L. H.; Hu, D. Y.; Liu, G. J. Fluorine Chem. 2015, 126, 87.
/
| 〈 |
|
〉 |