

栀子酰胺A-他克林杂合体的设计合成及其对6-羟多巴胺诱导的PC12细胞损伤的保护作用
收稿日期: 2016-11-22
修回日期: 2017-03-01
网络出版日期: 2017-03-03
基金资助
广东省自然科学基金(No.2015A030311012)和江西省自然科学基金(No.20151BAB215030)资助项目.
Design Synthesis of Gardenamide A/Tacrine Hybrids and Their Protection against 6-Hydroxydopamine-Induced Insults in PC12 Cells
Received date: 2016-11-22
Revised date: 2017-03-01
Online published: 2017-03-03
Supported by
Project supported by the Natural Science Foundation of Guangdong Province (No. 2015A030311012) and the Natural Science Foundation of Jiangxi Province (No. 20151BAB215030).
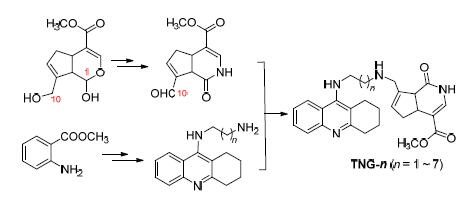
设计合成了7个栀子酰胺A (GA)-他克林杂合物. 首先以京尼平为原料,经5步反应合成10-栀子酰胺A醛(DG),总收率20.4%;再以邻氨基苯甲酸甲酯为原料,经4步反应合成N-末端氨基烷基他克林(TN-n),总收率50.0%~65.4%;随后,DG与TN-n在合适的还原剂作用下还原胺化获得目标化合物,收率37%~54%. 所有目标产物的结构均由1H NMR,13C NMR和ESI-MS确证. 同时建立了六羟多巴胺(6-OHDA)诱导损伤的PC12细胞模型对系列杂合物进行生物活性评价. 发现杂合物TNG-1和TNG-5具有比他克林、GA及其联合用药更好的神经保护作用,推测这两个杂合物可能具有比单体药物更好的类药性.
张馨怡 , 袁盛 , 赵家强 , 张磊 , 张潮 , 王日康 , 陈河如 . 栀子酰胺A-他克林杂合体的设计合成及其对6-羟多巴胺诱导的PC12细胞损伤的保护作用[J]. 有机化学, 2017 , 37(4) : 873 -880 . DOI: 10.6023/cjoc201611026
Seven gardenamide A (GA)/tacrine hybrids have been designed and synthesized. Firstly, 10-gardenamide A (DG) aldehyde was prepared through 5 steps reactions applied genipin as starting material. The overyield was 20.4%. Then, N-terminated aminoalkyltacrine (TN-n) was synthesized via 4 steps reactions started from o-aminobenzoic acid. The overall yield was 50.0%~65.4%. After that, DG reacted with TN-n at the presence of appropriate reductant leading to the target compounds. The yields were from 37% to 54%. The structures of all the target compounds were confirmed by 1H NMR, 13C NMR and ESI-MS. The bioactivities of all the hybrids were evaluated by setting up 6-hydroxydopamine-induced impaired PC12 cell model. It was indicated that two hybrids TNG-1 and TNG-5 showed better neuroprotective activity than tacrine, GA or their combination. Based on this fact, it was proposed that the hybrids TNG-1 and TNG-5 were better drug-like candidates than both monomers.

Key words: drug design; drug synthesis; gardenamide A; tacrine; alzheimer disease
[1] Zimmermann, G. R.; Lehar, J.; Keith, C. T. Drug Discovery Today 2007, 12, 34.
[2] Hanauske, A. R.; Chen, V.; Peoletti, P. Oncologist 2001, 6, 363.
[3] Graul, A.; Leeson, P.; Castaner, J. Drugs Future 1999, 24, 269.
[4] Sorbera, L. A.; Castaner, J.; Fresno, M. Drugs Future 2002, 27, 132.
[5] Geldenhuys, W. J.; Youdim, M. B. H.; Carroll, R. T.; Van der Schyf, C. J. Prog. Neurobiol. 2011, 94, 347.
[6] Pang, Y. P.; Cusack, B.; Groshan, K.; Richelson, E. J. Biol. Chem. 1996, 271, 15060.
[7] Bolognesi, M. L.; Cavalli, A.; Valgimigli, L. J. Med. Chem. 2007, 50, 6446.
[8] Fernandez, M. I.; Perez, C.; Campillo, N. E. ChemMedChem 2009, 4, 828.
[9] Luo, J.; Wang, J.; Huang, Z.; Yang, J.; Yao, X.; Chen, H.; Zheng, W. ChemMedChem 2012, 7, 1661.
[10] Wang, J.; Yang, J.; Peng, L.; Zhao, J.; Mu, N.; Huang, J.; Lazarovici, P.; Chen, H.; Zheng, W. Neuroscience 2015, 286, 242.
[11] Wang, J.; Peng, L.; Zhao, J.; Zhang, L.; Guo, C.; Zheng, W.; Chen, H. Int. J. Mol. Sci. 2015, 16, 22350.
[12] Dess, D. B.; Martin, J. C. J. Org. Chem. 1983, 48, 4155.
[13] Dess, D. B.; Martin, J. C. J. Am. Chem Soc.. 1991, 113, 7277.
[14] Carlier, P. R.; Han, Y. F.; Chow, E. S.-H.; Li, C. P.-L.; Wang, H.; Lieu, T. X.; Wong, H. S.; Pang, Y.-P. Bioorg. Med. Chem. 1999, 7, 351.
[15] Zhou, J. J.; Huang, H.; Xie, S. Q. Chin. Chem. Lett. 2008, 19, 99.
[16] Sheibley, F. E. J. Chem. Educ. 1943, 20, 115.
/
| 〈 |
|
〉 |