

季铵盐作为胺源经由两次C-N键活化的酯的胺解反应(英文)
收稿日期: 2016-12-06
修回日期: 2017-02-13
网络出版日期: 2017-03-03
基金资助
国家自然科学基金(No.21462031)、国家重点基础研究发展计划前期专项(No.2014CB460609)、内蒙古自治区高等学校科技项目基金(No.NJZZ14032)资助项目.
Aminolysis of Esters Using Quaternary Ammonium Salts as Amine Sources via Twice C-N Bond Activations
Received date: 2016-12-06
Revised date: 2017-02-13
Online published: 2017-03-03
Supported by
Project supported by the National Natural Science Foundation of China (No. 21462031), the Initial Special Research for National Basic Research Program of China (No. 2014CB460609) and the Research Program of Science and Technology at University of Inner Mongolia Autonomous Region (No. NJZZ14032).
王丽丽 , 李鹏帅 , 贾美林 , 包永胜 . 季铵盐作为胺源经由两次C-N键活化的酯的胺解反应(英文)[J]. 有机化学, 2017 , 37(5) : 1220 -1230 . DOI: 10.6023/cjoc201612014
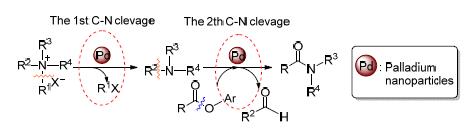
Catalyzed by supported palladium nanoparticles, an aminolysis reaction between various aryl esters and quaternary ammonium salts via twice C-N bond activations has been developed for selectively synthesis of amides. The Pd/γ-Al2O3 catalyst exhibited an excellent catalytic activity and reusability of at least five recycles in air for the reaction. The experiment results indicated that the first C-N cleavage of quaternary ammonium salt affords the tertiary amine and halohydrocarbon, and the second C-N cleavage proceeds via the formation of an iminium intermediate.
Key words: quaternary ammonium salts; aminolysis; palladium; C-N activation; aryl esters
[1] (a) Pattabiraman, V. R.; Bode, J. W. Nature 2011, 480, 471.
(b) Allen, C. L.; Williams, J. M. J. Chem. Soc. Rev. 2011, 40, 3405.
(c) Valeur, E.; Bradley, M. Chem. Soc. Rev. 2009, 38, 606.
[2] Bai, C. H.; Yao, X. F.; Li, Y. W. ACS Catal. 2015, 5, 884.
[3] (a) Nekkanti, S.; Veeramani, K.; Kumar, N. P.; Shankaraiah, N. Green chem. 2016, 18, 3439.
(b) Wu, K.; Huang, Z. L.; Ma, Y. Y.; Lei, A. W. RSC Adv. 2016, 6, 24349.
[4] Arefi, M.; Saberi, D.; Karimi, M.; Heydari, A. ACS Comb. Sci. 2015, 17, 341.
[5] Ouyang, K.; Hao, W.; Zhang, W. X.; Xi, Z. Chem. Rev. 2015, 115, 12045.
[6] (a) Bao, Y. S.; Baiyin, M.; Agula, B.; Jia, M. L.; Bao, Z. J. Org. Chem. 2014, 79, 6715.
(b) Bao, Y. S.; Bao, Z.; Agula, B.; Baiyin, M.; Jia, M. L. J. Org. Chem. 2014, 79, 803.
[7] (a) Li, Y. M.; Jia, F.; Li, Z. P. Chem.-Eur. J. 2012, 18, 5150.
(b) Porcheddu, A.; Luca, L. D. Adv. Synth. Catal. 2012, 354, 2949.
[8] Xu, K.; Hu, Y. B.; Zhang, S.; Zha, Z. G.; Wang, Z. Y. Chem.-Eur. J. 2012, 18, 9793.
[9] Du, B.; Sun, P. Sci. China Chem. 2014, 57, 1176.
[10] Gao, L.; Tang, H.; Wang, Z. Chem. Commun. 2014, 50, 4085.
[11] (a) Cassar, L.; Foa, M.; Gardano, A. J. Organomet. Chem. 1976, 121, C55.
(b) Bhardwaj, M.; Sahi, S.; Mahajan, H.; Paul, S.; Clark, J. H. J. Mol. Catal. A: Chem. 2015, 408, 48.
(c) Zhang, J. T.; Li, D. Y.; Chen, H.; Wang, B. J.; Liu, Z. X.; Zhang, Y. H. Adv. Synth. Catal. 2016, 358, 792.
[12] (a) Wang, X.; Zhu, L. Z.; Chen, S. H.; Xu, X. H.; Au, C. T.; Qiu, R. Org. Lett. 2015, 17, 5228.
(b) Lin, C. L.; Li, D.; Wang, Y. B.; Yao, J. Z.; Zhang, Y. H. Org. Lett. 2015, 17, 1328.
(c) Fabrizi, G.; Goggiamani, A.; Sferrazza, A.; Cacchi, S. Angew. Chem., Int. Ed. 2010, 49, 4067.
(d) Thirupathi, N.; Puri, S.; Reddy, T. J.; Sridhar, B.; Reddya, M. S. Adv. Synth. Catal. 2016, 358, 303.
(e) Xu, P.; Han, F. S.; Wang, Y. H. Adv. Synth. Catal. 2015, 357, 3441.
(f) Gadge, S. T.; Khedkar, M. V.; Lanke, S. R.; Bhanage, B. M. Adv. Synth. Catal. 2012, 354, 2049.
[13] (a) Kim, H. J.; Kim, J.; Cho, S. H.; Chang, S. J. Am. Chem. Soc. 2011, 133, 16382.
(b) Xue, Q. C.; Xie, J.; Li, H. M.; Cheng, Y. X.; Zhu, C. J. Chem. Commun. 2013, 49, 3700.
(c) Zhao, D.; Wang, T.; Shen, Q.; Li, J. X. Chem. Commun. 2014, 50, 4302.
(d) Wei, W.; Zhang, C.; Xu, Y.; Wan, X. B. Chem. Commun. 2011, 47, 10827.
(e) Liu, L. H.; Yun, L.; Wang, Z. K.; Fu, X. F.; Yan, C. H. Tetrahedron Lett. 2013, 54, 5383.
(f) Li, D. J.; Yang, T. H.; Su, H. L.; Yu, W. Adv. Synth. Catal. 2015, 357, 2529.
(g) Hao, W. J.; Du, Y.; Wang, D.; Jiang, B.; Gao, Q.; Tu, S. J.; Li, G. G. Org. Lett. 2016, 18, 1884.
(h) Zhang, H.; Dong, D. Q.; Hao, S. H.; Wang, Z. L. RSC Adv. 2016, 6, 8465.
(i) Tan, B.; Toda, N.; Barbas, C. F. Angew. Chem., Int. Ed. 2012, 51, 12538.
(j) Sun, J. W.; Wang, Y.; Pan, Y. J. Org. Chem. 2015, 80, 8945.
(k) Liu, Z.; Zhang, J.; Chen, S.; Shi, E.; Xu, Y.; Wan, X. Angew. Chem., Int. Ed. 2012, 51, 3231.
(l) Uyanik, M.; Okamoto, H.; Yasui, T.; Ishihara, K. Science 2010, 328, 1376.
[14] (a) Hirao, T.; Yamada, N.; Ohshiro, Y.; Agawa, T. J. Organomet. Chem. 1982, 236, 409.
(b) Hosomi, A.; Hoashi, K.; Kohra, S.; Tominaga, Y.; Otaka, K.; Sakurai, H. J. Chem. Soc., Chem. Commun. 1987, 570.
[15] (a) Wenkert, E.; Han, A.-L.; Jenny, C.-J. J. Chem. Soc., Chem. Commun. 1988, 975.
(b) Reeves, J. T.; Fandrick, D. R.; Tan, Z.; Song, J. J.; Lee, H.; Yee, N. K.; Senanayake, C. H. Org. Lett. 2010, 12, 4388.
[16] Blakey, S. B.; MacMillan, D. W. C. J. Am. Chem. Soc. 2003, 125, 6046.
[17] (a) Xie, L.-G.; Wang, Z.-X. Angew. Chem., Int. Ed. 2011, 50, 4901.
(b) Zhang, X.-Q.; Wang, Z.-X. J. Org. Chem. 2012, 77, 3658.
[18] Zhang, X. Q.; Wang, Z.-X. Org. Biomol. Chem. 2014, 12, 1448.
[19] (a) Lei, Y.; Zhang, R.; Wu, L.; Wu, Q.; Mei, H.; Li, G. Appl. Organomet. Chem. 2014, 28, 310.
(b) Lei, Y.; Zhang, R.; Wu, Q.; Mei, H.; Xiao, B.; Li, G. J. Mol. Catal. A: Chem. 2014, 381, 120.
[20] Rozita, Y.; Brydson, R.; Comyn, T. P.; Scott, A. J.; Hammond, C.; Brown, A.; Chauruka, S.; Hassanpour, A.; Young, N. P.; Kirkland, A. I. ChemCatChem 2013, 5, 2695.
[21] Halima, T. B.; Zhang, W.; Yalaoui, I.; Hong, X.; Yang, Y.; Houk, K. N.; Newman, S. G. J. Am. Chem. Soc. 2017, 139, 1311.
[22] Sun, J. W.; Fu, Y. S.; He, G. Y.; Sun, X. Q.; Wang, X. Catal. Sci. Technol. 2014, 4, 1742.
[23] Pillo, T.; Zimmermann, R.; Steiner, P.; Hüfner, S. J. Phys.: Condens. Matter 1997, 9, 3987.
[24] Murata, S.; Miura, M.; Nomura, M. J. Org. Chem. 1989, 54, 4700.
[25] (a) North, M. Angew. Chem., Int. Ed. 2004, 43, 4126.
(b) Murahashi, S. I.; Komiya, N.; Terai, H.; Nakae, T. J. Am. Chem. Soc. 2003, 125, 15312.
(c) Murahashi, S. I.; Komiya, N.; Terai, H. Angew. Chem., Int. Ed. 2005, 44, 6931.
[26] Guo, S. M.; Qian, B.; Xie, Y. J.; Xia, C. G.; Huang, H. M. Org. Lett. 2011, 13, 522.
[27] Zhang, D. L.; Bao, Z.; Bao, Y. S. J. Phys. Chem. C 2015, 119, 20426.
[28] Naumov, Y. A.; Dremova, V. P.; Kost, A. N.; Mentus, A. N.; Smirnova, S. N. Tr. Vses. Nauch.-Issled. Inst. Dezinfek. Steriliz. 1970, 2, 24.
[29] Houghton, R. P.; Williams, C. S. Tetrahedron Lett. 1967, 40, 3929.
[30] Kushner, S.; Dalalian, H.; Sanjurjo, J. L.; BachJr, F. L.; Safir, S. R.; SmithJr, V. K.; Williams, J. H. J. Am. Chem. Soc. 1952, 74, 3617.
[31] Chow, C. T.; Chi, J. Y. Acta Chim. Sinica 1962, 28, 236 (in Chinese). (周啓霆, 嵇汝运, 化学学报, 1962, 28, 236.)
[32] Wu, K.; Huang, Z. L.; Ma, Y. Y.; Lei, A. W. RSC Adv. 2016, 6, 24349.
[33] Pathak, U.; Bhattacharyya, S.; Pandey, L. K.; Mathur, S.; Jain, R. RSC Adv. 2014, 4, 3900.
[34] Fang, T.; Gao, X. H.; Tang, R. Y.; Zhang, X. G.; Deng, C. L. Chem. Commun. 2014, 50, 14775.
[35] Bao, Y. S.; Bao, A.; Bao, Z.; Bai, Y.; Jia, M. J. Org. Chem. 2014, 79, 803.
[36] Bhattacharya, A.; Plata, R. E.; Villarreal, V.; Muramulla, S.; Wu, J. Tetrahedron Lett. 2006, 47, 505.
[37] Reay, A. J.; Fairlamb, I. J. S. Chem. Commun. 2015, 51, 16289.
/
| 〈 |
|
〉 |