

C-H…O氢键驱动的1,2,3-三氮唑折叠体:评估分子间C-H…X-(X=Cl,Br,I)和C-H…N氢键的稳定性
收稿日期: 2017-02-11
修回日期: 2017-03-07
网络出版日期: 2017-03-17
基金资助
国家自然科学基金(Nos.21272042,21432004)资助项目.
Intramolecular C-H…O Hydrogen Bonding-Driven 1,2,3-Trazole Foldamers:Assessment of Intermolecular C-H…X- (X=Cl, Br, I) and C-H…N Hydrogen Bonding
Received date: 2017-02-11
Revised date: 2017-03-07
Online published: 2017-03-17
Supported by
Project supported by the National Natural Science Foundation of China (Nos.21272042,21432004).
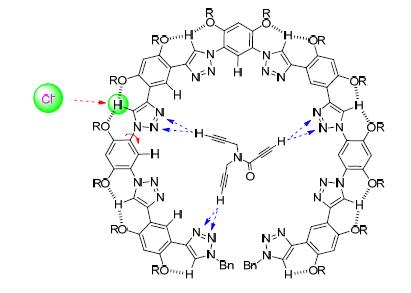
1,2,3-三氮唑芳香寡聚体可以通过分子内三中心C-H…O氢键诱导形成折叠或螺旋二级结构.通过1H NMR实验研究这类人工二级结构在氯仿和二氯甲烷中进一步形成分子间C-H…Cl-和C-H…N氢键的倾向性,发现分子内的两类C-H…O氢键可以通过进一步形成C-H…Cl-氢键而被弱化.在过量Cl-存在时,三氮唑N-1侧的六元环C-H…O氢键被显著破坏,由此形成分子间C-H…Cl-氢键,从而诱导骨架形成另一类更加扩展的折叠构象.过量的Br-和I-也可以形成类似的分子间氢键.对其中一个八聚体研究揭示,1,2,3-三氮唑螺旋体的内侧2,3-位N原子还可以与三炔和二炔衍生物的炔基C-H形成分子间弱的C-H…N氢键,三氮唑折叠结构通过诱导N原子形成环形定位促进这一分子间弱氢键产生协同效应.
孙广军 , 聂承斌 , 赵新 , 黎占亭 . C-H…O氢键驱动的1,2,3-三氮唑折叠体:评估分子间C-H…X-(X=Cl,Br,I)和C-H…N氢键的稳定性[J]. 有机化学, 2017 , 37(7) : 1757 -1763 . DOI: 10.6023/cjoc201702012
1,2,3-Triazole aromatic oligomers are driven by intramolecular three-center C-H…O hydrogen bonding to form folded or helical secondary structures. This paper reports the assessment of their ability to form intermolecular C-H…Cl- in CDCl3 or C-H…N hydrogen bonding in CD2Cl2 by using 1H NMR. It is revealed that the two kinds of intramolecular six-membered C-H…O hydrogen bondings of the backbones are both weakened by Cl- through the formation of intermolecular C-H…Cl- hydrogen bonding. In the presence of excess of Cl-, the C-H…O hydrogen bonding on the N-1 side of the triazole units is, to a large extent, broken by intermolecular C-H…Cl- hydrogen bonding, which induces the backbones to form another kind of more extended crescent secondary structures. Under similar conditions, excess of Br- and I- can also form similar intermolecular hydrogen bonding. It is also found that the inside-located N-2 and N-3 atoms of the triazole units of a 8-mer oligomer can also form weak intermolecular C-H…N hydrogen bonding with C-H atoms of the alkynyl units of several tri-and bi-alkynes, which is enhanced by the folded conformation of the oligomer through forcing the N-2 and N-3 atoms to arrange into a ring.

[1] Hill, D. J.; Mio, M. J.; Prince, R. B.; Hughes, T. S.; Moore, J. S. Chem. Rev. 2001, 101, 3893.
[2] Huc, I. Eur. J. Org. Chem. 2004, 17.
[3] Gong, B. Acc. Chem. Res. 2008, 41, 1376.
[4] Li, Z.-T.; Hou, J.-L.; Li, C. Acc. Chem. Res. 2008, 41, 1343.
[5] Juwarker, H.; Suk, J.-M.; Jeong, K.-S. Chem. Soc. Rev. 2009, 38, 3316.
[6] Ni, B.-B.; Yan, Q.; Ma, Y.; Zhao, D. Coord. Chem. Rev. 2010, 254, 954.
[7] Gan, Q.; Wang, Y.; Jiang, H. Curr. Org. Chem. 2011, 15, 1293.
[8] Zhang, D.-W.; Zhao, X.; Hou, J.-L.; Li, Z.-T. Chem. Rev. 2012, 112, 5271.
[9] Zhang, D.-W.; Zhao, X.; Li, Z.-T. Acc. Chem. Res. 2014, 47, 1961.
[10] Lee, S.; Flood, A. H. Top. Heterocycl. Chem. 2012, 28, 85.
[11] Hua, Y.; Flood, A. H. Chem. Soc. Rev. 2010, 39, 1262.
[12] Shi, Z.; Song, Y.; Lu, F.; Zhou, T.; Zhao, X.; Zhang, W.; Li, Z. Acta Chim. Sinica 2013, 71, 51(in Chinese). (施朱名, 宋宇, 陆方, 周天佑, 赵新, 张文科, 黎占亭, 化学学报, 2013, 71, 51.)
[13] Wang, Y.; Wang, H.; Zhang, D.; Li, Z. Chin. J. Org. Chem. 2016, 36, 1580(in Chinese). (汪奕, 王辉, 张丹维, 黎占亭, 有机化学, 2016, 36, 1580.)
[14] Zhang, Y.-C.; Chen, L.; Wang, H.; Zhou, Y.-M.; Zhang, D.-W.; Li, Z.-T. Chin. Chem. Lett. 2016, 27, 817.
[15] Chen, L.; Zhang, Y.-C.; Wang, W.-K.; Tian, J.; Zhang, L.; Wang, H.; Zhang, D.-W.; Li, Z.-T. Chin. Chem. Lett. 2015, 26, 811.
[16] Gan, Q.; Wang, Y.; Jiang, H. Chin. J. Chem. 2013, 31, 651.
[17] Meudtner, R. M.; Ostermeier, M.; Goddard, R.; Limberg, C.; Hecht, S. Chem. Eur. J. 2007, 13, 9834.
[18] Hua, Y.; Flood, A. H. J. Am. Chem. Soc. 2010, 132, 12838.
[19] Wang, Y.; Xiang, J.; Jiang, H. Chem. Eur. J. 2011, 17, 613.
[20] Wang, Y.; Bie, F.; Jiang, H. Org. Lett. 2010, 12, 3630.
[21] Wang, Y.; Li, F.; Han, Y.; Wang, F.; Jiang, H. Chem. Eur. J. 2009, 15, 9424.
[22] Zhu, Y.-Y.; Wang, G.-T.; Wang, R.-X.; Li, Z.-T. Cryst. Growth Des. 2009, 9, 4778.
[23] Lu, B.-Y.; Li, Z.-M.; Zhu, Y.-Y.; Zhao, X.; Li, Z.-T. Tetrahedron 2012, 68, 8857.
[24] Wu, C.-F.; Zhao, X.; Lan, W.-X.; Cao, C.; Liu, J.-T.; Jiang, X.-K.; Li, Z.-T. J. Org. Chem. 2012, 77, 4261.
[25] You, L.-Y.; Chen, S.-G.; Zhao, X.; Liu, Y.; Lan, W.-X.; Zhang, Y.; Lu, H.-J.; Cao, C.-Y.; Li, Z.-T. Angew. Chem., Int. Ed. 2012, 51, 1657.
[26] Wu, C.-F.; Li, Z.-M.; Xu, X.-N.; Zhao, Z.-X.; Zhao, X.; Wang, R.-X.; Li, Z.-T. Chem. Eur. J. 2014, 20, 1418.
[27] Liu, Y.-H.; Zhang, L.; Xu, X.-N.; Li, Z.-M.; Zhang, D.-W.; Zhao, X.; Li, Z.-T. Org. Chem. Front. 2014, 1, 494.
[28] Wang, D.-Y.; You, L.-Y.; Wang, J.-L.; Wang, H.; Zhang, D.-W.; Li, Z.-T. Tetrahedron Lett. 2013, 54, 6967.
[29] Holub, J. M.; Kirshenbaum, K. Chem. Soc. Rev. 2010, 39, 1325.
[30] Milli, L.; Larocca, M.; Tedesco, M.; Castellucci, N.; Ghibaudi, E.; Cornia, A.; Calvaresi, M.; Zerbetto, F.; Tomasini, C. J. Org. Chem. 2014, 79, 5958.
[31] Kann, N.; Johansson, J. R.; Beke-Somfai, T. Org. Biomol. Chem. 2015, 13, 2776.
[32] Li, X. Chem. Asian J. 2011, 6, 2606.
[33] Xu, Y.-Y.; Qian, A.-R.; Cao, X.-F.; Ling, C.-Y.; Cao, Y.-B.; Wang, R.-L.; Li, Y.-S.; Yang, Y.-S. Chin. Chem. Lett. 2016, 27, 703.
[34] Wang, B.; Shi, Y.; Zhan, Y.; Zhang, L.; Zhang, Y.; Wang, L.; Zhang, X.; Li, Y.; Li, Z.; Li, B. Chin. J. Chem. 2015, 33, 1124.
[35] Cai, M.; Hu, J.; Tian, J.-L.; Yan, H.; Zheng, C.-G.; Hu, W.-L. Chin. Chem. Lett. 2015, 26, 675.
[36] Desiraju, G. R. Acc. Chem. Res. 2002, 35, 565.
[37] Lehane, K. N.; Moynihan, E. J. A.; Brondel, N.; Lawrence, S. E.; Maguire, A. R. CrystEngComm 2007, 9, 1041.
[38] Yoosaf, K.; Llanes-Pallas, A.; Marangoni, T.; Belbakra, A.; Marega, R.; Botek, E.; Champagne, B.; Bonifazi, D.; Armaroli, N. Chem. Eur. J. 2011, 17, 3262.
/
| 〈 |
|
〉 |