

铁催化烯基芳烃的氢芳基化、甲基芳基化和二芳基化制备非对称1,1-二芳基烷烃的反应(英文)
收稿日期: 2017-02-25
修回日期: 2017-03-25
网络出版日期: 2017-03-31
基金资助
国家自然科学基金(Nos.21402200,21502191,21672213,21232001),中国科学院战略先导科技专项(No.XDB20000000)资助项目.
Iron Catalyzed Oxidative Hydroarylation, Methylarylation, and Diarylation of Vinylarenes to Generate Unsymmetrical 1,1-Diarylalkanes
Received date: 2017-02-25
Revised date: 2017-03-25
Online published: 2017-03-31
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21402200, 21502191, 21672213, 21232001), the Strategic Priority Research Program of Chinese Academy of Sciences (No. XDB20000000).
Kaki Raveendra Babu , 陈绍维 , 李亚军 , 鲍红丽 . 铁催化烯基芳烃的氢芳基化、甲基芳基化和二芳基化制备非对称1,1-二芳基烷烃的反应(英文)[J]. 有机化学, 2017 , 37(5) : 1160 -1164 . DOI: 10.6023/cjoc201702036
A novel iron catalyzed hydroarylation, methylarylation, and diarylation of styrenes to form unsymmetrical 1,1-diarylalkanes with electron rich anisole and 1,3,5-trimethoxybenzene under mild conditions have been developed. Benzoyl peroxide is used as an oxidant for hydroarylation, whereas in the case of methylarylation and diarylation the oxidant tert-butyl peracetate is used.
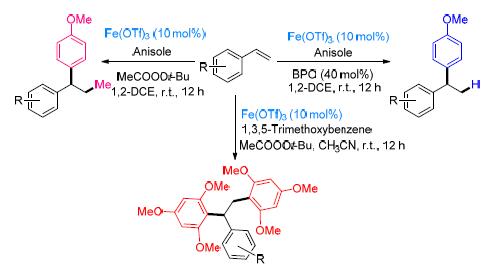
Key words: hydroarylation; methylarylation; diarylation; vinylarenes; 1,1-diarylalkanes
[1] (a) Weissermel, K.; Arpe, H. J. Industrielle Organische Chemie, 5th ed., Wiley-VCH, Weinheim, Germany, 1988, p. 167.
(b) Perego, C.; Ingallina, P. Catal. Today 2002, 73, 3.
(c) Perego, C.; Ingallina, P. Green Chem. 2004, 6, 274.
[2] For selected bioactive 1,1-diarylethanes, see: (a) Alami, M.; Messaoudi, S.; Hamze, A.; Provot, O.; Brion, J.-D.; Liu, J.-M.; Bignon, J.; Bakala, J. WO 2009/147217, 2009
[Chem. Abstr. 2009, 152, 37275].
(b) Moree, W. J.; Li, B.-F.; Jovic, F.; Coon, T.; Yu, J.; Gross, R. S.; Tucci, F.; Marinkovic, D.; Zamani-Kord, S.; Malany, S.; Bradbury, M. J.; M.Hermandez, L.; O'Brien, Z.; Wen, J.; Wang, H.; Hoare, S. R. J.; Petroski, R. E.; Sacaan, A.; Madan, A.; Crowe, P. D.; Beaton, G. J. Med. Chem. 2009, 52, 5307.
(c) Beaton, G.; Moree, W. J.; Jovic, F.; Coon, T.; Yu, J. US 2006/0014797, 2006
[Chem. Abstr. 2006, 144, 128847].
(d) Chubb, N. A. L.; Cox, M. R.; Dauvergne, J. S.; Ewin, R. A.; Lauret, C. US 0167506, 2007
[Chem. Abstr. 2007, 147, 189176].
(e) Pathak, T. P.; Osiak, J. G.; Vaden, R. M.; Welm, B. E.; Sigman, M. S. Tetrahedron 2012, 68, 5203.
(f) Messaoudi, S.; Hamze, A.; Provot, O.; Treguier, B.; Dé Losada, J. R.; Bignon, J.; Liu, J.-M.; Wdzieczak-Bakala, J.; Thoret, S.; Dubois, J.; Brion, J.-D.; Alami, M. ChemMedChem 2011, 6, 488.
(g) Wang, Z.; Ai, F.; Wang, Z.; Zhao, W.; Zhu, G.; Lin, Z.; Sun, J. J. Am. Chem. Soc. 2015, 137, 383.
[3] For selected bioactive other 1,1-diarylalkanes, see: (a) Silva, D. H. S.; Davino, S. C.; de Moraes Barros S. B.; Yoshida, M. J. Nat. Prod. 1999, 62, 1475.
(b) Hills, C. J.; Winter, S. A.; Balfour, J. A. Drugs 1998, 55, 813.
(c) Funaoka, M. Polym. Int. 1998, 47, 277.
(d) Stelmach, J. E.; Keith, R.; Parmee, E. R.; Tata, J. R. WO 2006/102067, 2006
[Chem. Abstr. 2006, 145, 377567].
(e) Kainuma, M.; Kasuga, J.-i.; Hosoda, S.; Wakabayashi, K.-i.; Tanatani, A.; Nagasawa, K.; Miyachi, H.; Makishima, M.; Hashimoto, Y. Bioorg. Med. Chem. Lett. 2006, 16, 3213.
(f) Kim, R. M.; Parmee, E. R.; Tan, Q.; Yang, C.-m.; Lins, A. R. WO 2007/136577, 2007
[Chem. Abstr. 2007, 148, 33513].
(g) Malhotra, B.; Gandelman, K.; Sachse, R.; Wood, N.; Michel, M. C. Curr. Med. Chem. 2009, 16, 4481.
(h) Hu, Q. Z.; Yin, L. N.; Jagusch, C.; Hille, U. E.; Hartmann, R. W. J. Med. Chem. 2010, 53, 5049.
(i) Babu, K. R.; Khan, F. A. Tetrahedron Lett. 2015, 56, 4067.
[4] (a) Botteghi, C.; Corrias, T.; Marchetti, M.; Paganelli, S.; Piccolo, O. Org. Proc. Res. Dev. 2002, 6, 379.
(b) Cheltsov, A. V.; Aoyagi, M.; Aleshin, A.; Yu, E. C.-W.; Gilliland, T.; Zhai, D.; Bobkov, A. A.; Reed, J. C.; Liddingtonand, R. C.; Abagyan, R. J. Med. Chem. 2010, 53, 3899.
(c) Ochsner, M.; Creba, J.; Walker, J.; Bentley, P.; Muakkassah-Kelly, S. F. Biochem. Pharmacol. 1990, 40, 2247.
(d) Tscheschet, R.; Delhvi, M. S.; Sepúlveda-Boza, S.; Zilliken, F.; Kirfeland, A.; Will, G. Liebigs Ann. Chem. 1985, 1985, 2465.
[5] Selected recent reviews on the synthesis of 1,1-diarylalkanes: (a) Chen, C.; Dong, X.-Q.; Zhang, X. Org. Chem. Front. 2016, 3, 1359.
(b) Mondal, S.; Panda, G. RSC Adv. 2014, 4, 28317.
[6] Selected papers for the synthesis of 1,1-diarylalkanes via difunctionalization of olefins: (a) Ouyang, X.-H.; Song, R.-J.; Hu, M.; Yang, Y.; Li, J.-H. Angew. Chem., Int. Ed. 2016, 55, 3187.
(b) Tang, E.; Zhao, Y.; Li, W.; Wang, W.; Zhang, M.; Dai, X. Org. Lett. 2016, 18, 912.
[7] Selected papers for the synthesis of 1,1-diarylalkanes via 1,1-diarylalkenes hydrogenation: (a) Chen, J.; Chen, C.; Ji, C.; Lu, Z. Org. Lett. 2016, 18, 1594.
(b) Wang, X.; Guram, A.; Caille, S.; Hu, J.; Preston, J. P.; Ronk, M.; Walker, S. Org. Lett. 2011, 13, 1881.
(c) Yan, Q.; Kong, D.; Zhao, W.; Zi, G.; Hou, G. J. Org. Chem. 2016, 81, 2070.
[8] Selected papers for the synthesis of 1,1-diarylalkanes via benzylic C-H functionalization: (a) Sha, S.-C.; Jiang, H.; Mao, J.; Bellomo, A.; Jeong, S. A.; Walsh, P. J. Angew. Chem., Int. Ed. 2016, 56, 1070.
(b) Ronson, T. O.; Carney, J. R.; Whitwood, A. C.; Taylor, R. J. K.; Fairlamb, I. J. S. Chem. Commun. 2015, 51, 3466.
[9] Selected papers for the synthesis of 1,1-diarylalkanes via Friedel-Crafts reactions: (a) Pallikonda, G.; Chakravarty, M. J. Org. Chem. 2016, 81, 2135.
(b) Niggemann, M.; Meel, M. J. Angew. Chem., Int. Ed. 2010, 49, 3684.
(c) Iovel, I.; Mertins, K.; Kischel, J.; Zapf, A.; Beller, M. Angew. Chem., Int. Ed. 2005, 44, 3913.
(d) Mertins, K.; Iovel, I.; Kischel, J.; Zapf, A.; Beller, M. Adv. Synth. Catal. 2006, 348, 691.
[10] Other methods for the 1,1-diarylalkanes synthesis: (a) Giedyk, M.; Goliszewska, K.; Ó Proinsias, K.; Gryko, D. Chem. Commun. 2016, 52, 1389.
(b) Guduguntla, S.; Hornillos, V.; Tessier, R.; Fañanás-Mastral, M.; Feringa, B. L. Org. Lett. 2016, 18, 252.
[11] (a) Wang, M. Z.; Wong, M. K.; Che, C. M. Chem. Eur. J. 2008, 14, 8353.
(b) Hu, X. B.; Martin, D.; Melaimi, M.; Bertrand, G. J. Am. Chem. Soc. 2014, 136, 13594.
[12] (a) Sun, H. B.; Li, B.; Hua, R. M.; Yin, Y. W. Eur. J. Org. Chem. 2006, 4231.
(b) Rueping, M.; Nachtsheim, B. J.; Scheidt, T. Org. Lett. 2006, 8, 3717.
[13] Hu, F.; Patel, M.; Luo, F. X.; Flach, C.; Mendelsohn, R.; Garfunkel, E.; He, H. X.; Szostak, M. J. Am. Chem. Soc. 2015, 137, 14473.
[14] Kischel, J.; Jovel, I.; Mertins, K.; Zapf, A.; Beller, M. Org. Lett. 2006, 8, 19.
[15] (a) Peregoand, C.; Ingallina, P. Green Chem. 2004, 6, 274.
(b) Peregoand, C.; Ingallina, P. Catal. Today 2002, 73, 3.
[16] Niggemannand, M.; Bisek, N. Chem. Eur. J. 2010, 16, 11246.
[17] Perez, M.; Mahdi, T.; Hounjetand, L. J.; Stephan, D. W. Chem. Commun. 2015, 51, 11301.
[18] (a) Anderson, L. L.; Arnold, J.; Bergman, R. G. J. Am. Chem. Soc. 2005, 127, 14542.
(b) Marcsekova, K.; Doye, S. Synthesis 2007, 145.
[19] (a) Mohan, D. C.; Patiland, R. D.; Adimurthy, S. Eur. J. Org. Chem. 2012, 3520.
(b) Cejka, J.; Wichterlova, B. Catal. Rev. Sci. Eng. 2002, 44, 375.
[20] Wen, J. Y.; Qi, H. F.; Kong, X. J.; Chen, L. G.; Yan, X. L. Synth. Commun. 2014, 44, 1893.
[21] Friis, S. D.; Pirnot, M. T.; Buchwald, S. L. J. Am. Chem. Soc. 2016, 138, 8372.
[22] Crisenza, G. E. M.; Sokolova, O. O.; Bower, J. F. Angew. Chem., Int. Ed. 2015, 54, 14866.
[23] (a) Joslin, E. E.; McMullin, C. L.; Gunnoe, T. B.; Cundari, T. R.; Sabat, M.; Myers, W. H. Organometallics 2012, 31, 6851.
(b) Foley, N. A.; Lee, J. P.; Ke, Z. F.; Gunnoe, T. B.; Cundari, T. R. Acc. Chem. Res. 2009, 42, 585.
(c) Lail, M.; Arrowood, B. N.; Gunnoe, T. B. J. Am. Chem. Soc. 2003, 125, 7506.
[24] (a) Luedtke, A. T.; Goldberg, K. I. Angew. Chem., Int. Ed. 2008, 47, 7694.
(b) Karshtedt, D.; Belland, A. T.; Tilley, T. D. Organometallics 2004, 23, 4169.
[25] Lee, S. Y.; Villani-Gale, A.; Eichman, C. C. Org. Lett. 2016, 18, 5034.
[26] Ma, Y.; Zhang, D.; Yan, Z.; Wang, M.; Bian, C.; Gao, X.; Bunel, E. E.; Lei, A. Org. Lett. 2015, 17, 2174.
[27] Tan, F.-L.; Song, R.-J.; Hu, M.; Li, J.-H. Org. Lett. 2016, 18, 3198.
[28] (a) Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Chem. Rev. 2011, 111, 1293.
(b) Bauer, I.; Knölker, H.-J. Chem. Rev. 2015, 115, 3170.
(c) Sherry, B. D.; Fürstner, A. Acc. Chem. Res. 2008, 41, 1500.
(d) Fürstner, A. Angew. Chem., Int. Ed. 2009, 48, 1364.
[29] (a) Li, Y.; Han, Y.; Xiong, H.; Zhu, N.; Qian, B.; Ye, C.; Kantchev, E. A.; Bao, H. Org. Lett. 2016, 18, 392.
(b) Zhu, N.; Zhao, J.; Bao, H. Chem. Sci. 2017, 8, 2081.
(c) Babu, K. R.; Zhu, N.; Bao, H. Org. Lett. 2017, 19, 46.
/
| 〈 |
|
〉 |