

4,4'-取代-2,2'-联吡啶钠和铕穴状化合物的合成和性质(英文)
收稿日期: 2017-02-03
修回日期: 2017-03-27
网络出版日期: 2017-04-01
基金资助
国家自然科学基金(No.21372171)资助项目.
Synthesis and Properties of Sodium and Europium(Ⅲ) Cryptates Incorporating the 4,4'-Substituted-2,2'-bipyridine Units
Received date: 2017-02-03
Revised date: 2017-03-27
Online published: 2017-04-01
Supported by
Project supported by the National Natural Science Foundation of China (No. 21372171).
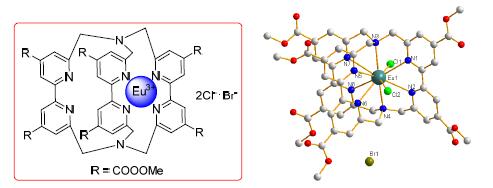
报道了由芳香醛合成4,4',6,6'-取代-2,2'-联吡啶化合物的方法,合成了一系列钠穴状化合物以及两个铕穴状化合物[Eu⊂bpy·bpy·bpy]·2Cl·Br(bpy=6,6'-二亚甲基-2,2'-联吡啶-4,4'-二甲酯)(23)和[Eu⊂bpy1·bpy1·bpy2]·2Cl·Br(bpy1=6,6'-二亚甲基-2,2'-联吡啶-4,4'-二甲酸,bpy2=6,6'-二亚甲基-2,2'-联吡啶-4,4'-二甲酰乙二胺)(24).目标化合物均经过1H NMR、13C NMR和高分辨质谱(HRMS)表征.X射线单晶衍射(XRD)和HRMS鉴定了铕穴状化合物23的结构.对23的荧光光谱(PL)、荧光衰减曲线和绝对量子产率(η)的研究结果表明,23能够有效地被近紫外光激发,显示荧光寿命(τ)为0.32 ms,η为70%的亮红色荧光,表明23是一种在发光和照明显示之中有着潜在应用的荧光材料.
陈素芳 , 洪玉标 , 刘元忠 , 薛明强 , 郑煜 , 沈琪 . 4,4'-取代-2,2'-联吡啶钠和铕穴状化合物的合成和性质(英文)[J]. 有机化学, 2017 , 37(5) : 1198 -1204 . DOI: 10.6023/cjoc201702004
A general strategy for the syntheses of 4,4',6,6'-substituted-2,2'-bipyridines starting from aromatic aldehyde is described. A series of sodium cryptates and two europium cryptates [Eu⊂bpy·bpy·bpy]·2Cl·Br (bpy=6,6'-dimethylene-2,2'-bipyridine-4,4'-dimethylester) (23) and [Eu⊂bpy1·bpy1·bpy2]·2Cl·Br (bpy1=6,6'-dimethylene-2,2'-bipyridine-4,4'-dicarboxylic acid, bpy2=6,6'-dimethylene-2,2'-bipyridine-4,4'-diformylethylenediamine) (24), were synthesized from these compounds. Sodium cryptates have been confirmed by 1H NMR, 13C NMR and high resolution mass spectrum (HRMS). The structure of europium cryptate 23 was confirmed by X-ray diffraction (XRD) and HRMS. The photoluminescence (PL) spectra, decay curve and the absolute quantum efficiency (η) of 23 are presented. 23 can be efficiently excited by near-UV light and presents a bright red luminescence with the lifetime (τ) of 0.32 ms and the η of 70%. It is suggested that 23 is expected to be a potential efficient phosphor for lighting and display.

Key words: cryptates; spectroscopy; macrocyclic compounds; phosphor; crystal structure
[1] (a) Parker, D. Coord. Chem. Rev. 2000, 205, 109.
(b) Xu, J. D.; Corneillie, T. M.; Moore, E. G.; Law, G.-L.; Butlin, N. G.; Raymond, K. N. J. Am. Chem. Soc. 2011, 133, 19900.
(c) Xi, P.; Xia, H. Y.; Zhao, F. L.; Chen, B. W. Mater. Lett. 2015, 160, 463.
(d) Chai, W. X.; Zhang, X. L.; Song, L.; Hong, M. W.; Shi, H. S.; Wang, C. Y.; Guo, J. Y.; Zheng, X. J.; Chen, G.; Shu, K. Y. Mater. Lett. 2015, 145, 4.
(e) Yang, L. G.; Yu, Y. Z.; Wang, X.; Zhu, M. L.; Gao, Q. Q.; Dai, Y. Q.; Bian, Y. Z. Inorg. Chem. Commun. 2016, 73, 30.
(f) Parbhakar, S.; Gupta, R.; Behera, J. N.; Hussain, F. Inorg. Chem. Commun. 2016, 72, 117.
(g) Liu, Z. H.; Yang, Y. K.; Sun, Z. Z.; Wu, C. F. Opt. Mater. 2016, 62, 1.
(h) Sheelam, A.; Ramanujam, K. Electrochim. Acta 2016, 216, 457.
[2] Havas, F.; Leygue, N.; Danel, M.; Mestre, B.; Galaup, C.; Picard, C. Tetrahedron 2009, 65, 7673.
[3] (a) Patah, A.; Baechle, J.; Grampp, G. Electrochim. Acta 2016, 219, 305.
(b) de Oliveira, T. C.; Santos, H. P.; Lahoud, M. G.; Franco, D. F.; Freire, R. O.; Dutra, J. D. L.; Cuin, A.; de Lima, J. F.; Marques, L. F. J. Lumin. 2017, 181, 196.
(c) Zhang, Y.; Petersen, J. L.; Milsmann, C. J. Am. Chem. Soc. 2016, 138, 13115.
(d) Baumgartner, Y.; Maximilian Klein, Y.; Constable, E. C.; Housecroft, C. E.; Willgert, M. RSC Adv. 2016, 6, 86220.
(e) Armelao, L.; Dell'Amico, D. B.; Bellucci, L.; Bottaro, G.; Labella, L.; Marchetti, F.; Samaritani, S. Polyhedron 2016, 119, 371.
(f) Zavakhina, M. S.; Samsonenko, D. G.; Dybtsev, D. N.; Argent, S. P.; Blake, A. J.; Schroder, M.; Fedin, V. P. Russ. Chem. Bull. 2015, 64, 2908.
[4] (a) Mukkala, V.-M.; Kankare, J. J. HeIv. Chim. Acta 1992, 75, 1578.
(b) Rodriguz-Ubis, J.-C.; Alpha, B.; Plancherel, D.; Lehn, J.-M. HeIv. Chim. Acta 1984, 67, 2264.
(c) Guillaumont, D.; Bazin, H.; Benech, J.-M.; Boyer, M.; Mathis, G. ChemPhysChem 2007, 8, 480.
(d) Havas, F.; Danel, M.; Galaup, C.; Tisnès, P.; Picard, C. Tetrahedron Lett. 2007, 48, 999.
(e) Weng, G. H.; Zhu, B.; Ye, Y.; Li, S. J. Chin. J. Org. Chem. 2015, 35, 309 (in Chinese). (翁官欢, 朱彬, 叶杨, 李世军, 有机化学, 2015, 35, 309.)
[5] (a) Bazin, H.; Trinquet, E.; Mathis, G. Rev. Mol. Biotechnol. 2002, 82, 233.
(b) Alpha, B.; Lehn, J.-M.; Mathis, G. Angew. Chem., Int. Ed. Engl. 1987, 26, 266.
(c) Prat, O.; Lopez, E.; Mathis, G. Anal. Biochem. 1991, 195, 283.
(d) Alpha, B.; Ballardini, R.; Balzani, V.; Lehn, J.-M.; Perathoner, S.; Sabbatini, N. Photochem. Photobiol. 1990, 52, 299.
[6] (a) Lopez, E.; Chypre, C.; Alpha, B.; Mathis, G. Clin. Chem. 1993, 39, 196.
(b) Brunet, E.; Juanes, O.; Rodriguez-Ubis, J. C. Curr. Chem. Biol. 2007, 1, 11.
(c) Nasso, I.; Galaup, C.; Havas, F.; Tisnes, P.; Picard, C.; Laurent, S.; Vander Elst, L.; Muller, R. N. Inorg. Chem. 2005, 44, 8293.
[7] KrÖHnke, F. Synthesis 1976, 1.
[8] Constable, E. C.; Redondo, A. H.; Housecroft, C. E.; Neuburger, M.; Schaffner, S. Dalton Trans. 2009, 6634.
[9] Harding, M. M.; Koert, U.; Lehn, J.-M.; Marquis-Rigault, A.; Piguet, C.; Siegel, J. HeIv. Chim. Acta 1991, 74, 594.
[10] Neises, B.; Steglich, W. Org. Synth. 1985, 63, 183.
[11] (a) Mangalum, A.; Morgan, B. P.; Hanley, J. M.; Jecen, K. M.; McGill, C. J.; Robertson, G. A.; Smith, R. C. Chem. Commun. 2010, 46, 5136.
(b) Liu, P. N.; Chen, Y. C.; Deng, J. G.; Tu, Y. Q. Synthesis 2001, 2078.
[12] Bischof, C.; Wahsner, J.; Scholten, J.; Trosien, S.; Seitz, M. J. Am. Chem. Soc. 2010, 132, 14334.
[13] Bkouche-Waksman, I.; Guilhem, J.; Pascard, C.; Alpha, B.; Deschenaux, R.; Lehn, J.-M. HeIv. Chim. Acta 1991, 74, 1163.
[14] Yuan, B. L.; Huang, Y. L.; Yu, Y. M.; Kim, S. I.; Seo, H. J. Mater. Lett. 2012, 70, 57.
[15] Peng, A. H.; Xie, E. Q.; Jia, C. W.; Jiang, R.; Lin, H. F. Mater. Lett. 2005, 59, 3866.
[16] Qi, S. Y.; Xie, H. D.; Huang, Y. L.; Kim, S. I.; Seo, H. J. Opt. Mater. Express 2014, 4, 190.
[17] Praveen Rao, P. N.; Amini, M.; Li, H. Y.; Habeeb, A. G.; Knaus, E. E. J. Med. Chem. 2003, 46, 4872.
[18] Karrer, P.; Cochand, C.; Neuss, N. Helv. Chim. Acta 1946, 29, 1836.
[19] Alpha, B.; Anklam, E.; Deschenaux, R.; Lehn, J-M.; Pietraskiewiez, M. Helv. Chim. Acta 1988, 71, 1042.
/
| 〈 |
|
〉 |