

手性双苯并噁嗪-2-酮二酚衍生物的高效合成及其在不对称催化中的应用
收稿日期: 2017-01-22
修回日期: 2017-03-14
网络出版日期: 2017-04-10
基金资助
国家自然科学基金(No.21502074)和江苏省高校自然科学重大研究(No.15KJA150006)资助项目.
Efficient and Stereoselective Synthesis of the Chiral Dihydroxy-bisquinazoline-dione Derivatives and Their Application in Asymmetric Catalysis
Received date: 2017-01-22
Revised date: 2017-03-14
Online published: 2017-04-10
Supported by
Project supported by the National Natural Science Foundation of China (No. 21502074) and the Major Basic Research Project of the Natural Science Foundation of the Jiangsu Higher Education Institutions (No. 15KJA150006).
林伟 , 蔡琦 , 郑纯智 , 黄志斌 , 史达清 . 手性双苯并噁嗪-2-酮二酚衍生物的高效合成及其在不对称催化中的应用[J]. 有机化学, 2017 , 37(8) : 2094 -2100 . DOI: 10.6023/cjoc201701044
An efficient and simple method for the synthesis of chiral benzo[5,6][1,3]oxazino[4,3-c]quinoxaline-dione is reported. 2-Hydroxybenzaldehydes and diamines were utilized as the starting materials, the formed diimines then underwent a reductive-cyclization process with triphosgene to give bis-quinazoline-dione derivatives induced by low-valent titanium reagent. Then through the conversion of functional groups, the bis-quinazoline-dione compounds containing hydroxy group have been obtained from their corresponding compounds containing methoxy group in the presence of BBr3. In addition, the dihydroxy-bis-quinazoline-dione derivatives were applied on the asymmetric nucleophilic addition of Et2Zn to aldehydes. The reported method is the efficient approach for the synthesis of dihydroxy-bis-quinazoline-dione derivatives.
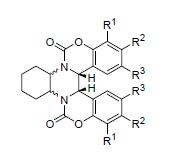
Key words: bis-quinazoline-dione; asymmetric; synthesis
[1] (a) Clardy, J.; Walsh, C. Nature 2004, 432, 829.
(b) Boger, D. L.; Boyce, C. W.; Labroli, M. A.; Sehon, C. A.; Jim, Q. J. Am. Chem. Soc. 1999, 121, 54.
(c) Riveiro, M. E.; Shayo, C.; Monczor, F.; Fernandez, N.; Baldi, A.; Kimpe, N. D.; Rossi, J.; Debenedetti, S.; Davio, C. Cancer Lett. 2004, 210, 179.
(d) Riveiro, M. E.; Maes, D.; Vazquez, R.; Vermeulen, M.; Mangelinckx, S.; Jacobs, J.; Debenedetti, S.; Shayo, C.; De Kimpe, N.; Davio, C. Bioorg. Med. Chem. 2009, 17, 6547.
(e) Cho, J. Y.; Hwang, T. L.; Chang, T. H.; Lim, Y. P.; Sung, P. J.; Lee, T. H.; Chen, J. J. Food Chem. 2012, 135, 17.
[2] Kung, P. P.; Casper, M. D.; Cook, K. L.; Wilson-Lingardo, L.; Risen, L. M.; Vickers, T. A.; Ranken, R.; Blyn, L. B.; Wyatt, J. R.; Cook, P. D.; Ecker, D. J. J. Med. Chem. 1999, 42, 4705.
[3] Malamas, M. S.; Millen, J. J. Med. Chem. 1991, 34, 1492.
[4] (a) Baek, D.; Park, Y.; Heo, H. I.; Lee, M.; Yang, Z.; Choi, M. Bioorg. Med. Chem. Lett. 1998, 8, 3287.
(b) Webber, S. E.; Bleckman, T. M.; Attard, J.; Deal, J. G.; Kathardekar, V. K.; Welsh, M.; Webber, S.; Janson, C. A.; Matthews, D. A.; Smith, W. W. J. Med. Chem. 1993, 36, 733.
[5] Hess, H. J.; Cronin, T. H.; Scriabine, A. J. Med. Chem. 1968, 11, 130.
[6] Chao, Q.; Deng, L.; Shih, H.; Leoni, L. M.; Genini, D.; Carson, D. A.; Cottam, H. B. J. Med. Chem. 1999, 42, 3860
[7] (a) Gibson, K. H.; Grundy, W.; Godfrey, A. A.; Woodburn, J. R.; Ashton, S. E.; Curry, B. J.; Scarlett, L.; Barker, A. J.; Brown, D. S. Bioorg. Med. Chem. Lett. 1997, 7, 2723.
(b) Myers, M. R.; Setzer, N. N.; Spada, A. P.; Zulli, A. L.; Hsu, C. J.; Zilberstein, A.; Johnson, S. E.; Hook, L. E.; Jacoski, M. V. Bioorg. Med. Chem. Lett. 1997, 7, 41.
[8] Veer, A. Biol. Pharm. Bull. 2003, 26, 1711.
[9] Jakobsen, P.; Ritsmar, P. B.; Persson, E. Bioorg. Med. Chem. 2000, 8, 2803.
[10] (a)Sun, H. S.; Wang, J. Q.; Tan, W. W.; Yang, H. D.; Li, Y. L.; Shen, L. J.; Guo, C. Chin. J. Org. Chem. 2016, 36, 622(in Chinese). (孙宏顺, 王建强, 谭文文, 杨海东, 李玉龙, 沈临江, 郭成, 有机化学, 2016, 36, 622.)
(b) Wu, J.; Kong, H. H.; Ding, M. W. Chin. J. Org. Chem. 2016, 36, 1662(in Chinese). (武静, 孔晗晗, 丁明武, 有机化学, 2016, 36, 1662.)
[11] (a) Ozaki, K.; Yamada, Y.; Oine, T.; Ishizuka, T.; Iwasawa, Y. J. Med. Chem. 1985, 28, 568.
(b) Argandona, V. H.; Luza, J. G.; Niemeyer, H. M. Phytochemistry 1980, 19, 1665.
(c) Erik, L.; Lars, P. C. J. Agric. Food Chem. 2000, 48, 2556.
(d) Hou, Z. K.; Ren, Y. G.; Huang, K. L. WO 2004087694, 2004[Chem. Abstr. 2004, 141, 194014].
[12] Yoo, C. L.; Fettinger, J. C.; Kurth, M. J. J. Org. Chem. 2005, 70, 6941.
[13] Coyne, W. E.; Cusic, J. W. J. Med. Chem. 1968, 11, 1208.
[14] Liu, J. F.; Kaselj, M.; Isome, Y.; Ye, P.; Sargent, K.; Sprague, K.; Cherrak, D.; Wilson, C. J.; Si, Y.; Yohannes, D.; Ng, S. C. J. Comb. Chem. 2006, 8, 7.
[15] Dou, G. L.; Shi, D. Q.; Li, Y. H. J. Comb. Chem. 2010, 12, 195.
[16] Lin, W.; Wang, Y. Z.; Zheng, Y. X.; Shi, D. Q. Heterocycles 2017, 94, 75.
[17] Tang, Z. L.; Xia, Z. W.; Li, X. X. Chin. J. Org. Chem. 2016, 36, 590(in Chinese). (唐子龙, 夏赞稳, 李新兴, 有机化学, 2016, 36, 590.)
[18] Lin, W.; Dou, G. L.; Hu, M. H.; Cao, C. P.; Huang, Z. B.; Shi, D. Q. Org. Lett. 2013, 15, 1238.
/
| 〈 |
|
〉 |