

α-烷氧酰胺亚甲基膦酸酯合成方法的研究
收稿日期: 2017-01-25
修回日期: 2017-03-08
网络出版日期: 2017-04-13
基金资助
国家自然科学基金(No.21462056)、贵州省联合基金(No.[2014]7542)、贵州省大学生创新(No.201610661006)资助项目.
Study of the Synthesis of α-Alkoxycarbonylaminomethylphosphonates
Received date: 2017-01-25
Revised date: 2017-03-08
Online published: 2017-04-13
Supported by
Project supported by the National Natural Science Foundation of China (No. 21462056), the Science and Technology Foundation of Guizhou Province (No.[2014]7542), and the Education Department of Guizhou Province (No. 201610661006).
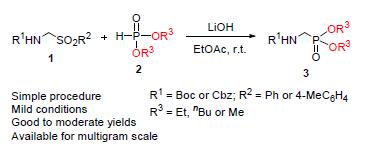
α-烷氧酰胺亚甲基膦酸酯由于亚甲基结构的特殊性使得其合成方法很有限,针对该问题研究了这一类型化合物的合成方法学.以乙酸乙酯作为溶剂,在氢氧化锂碱性条件下,α-芳磺酰亚甲基氨基甲酸酯与磷酸二酯反应能以良好至中等的产率顺利地合成常见方法难以合成的α-烷氧酰胺亚甲基膦酸酯.该方法操作简便,条件温和,并可应用于克级规模反应.
关键词: α-烷氧酰胺亚甲基膦酸酯; α-磺酰亚甲基氨基甲酸酯; 膦酸酯
黄晓丽 , 任林静 , Sajjad Ali , 蒲家志 , 姚秋丽 . α-烷氧酰胺亚甲基膦酸酯合成方法的研究[J]. 有机化学, 2017 , 37(8) : 2073 -2077 . DOI: 10.6023/cjoc201701047
Methods for the synthesis of α-alkoxycarbonylaminomethylphosphonates are very limited because of the presence of unique methylene group in their structures. Thus the methodology for the synthesis of this kind of compounds is studied. Under the basic conditions of using LiOH, α-sulfonylmethylcarbamates reacted with dialkylphosphonates in the solvent of commercial EtOAc giving the products of α-alkoxycarbonylaminomethylphosphonates which are typically hard to be synthesized in good to moderate yields. This simple protocol runs under very mild conditions, and can be exploited for multigram amounts with good yield too.

[1] Kukuhar, V. P.; Hudson, H. R. Aminophosphorus Acids and Aminophosphinic Acids, Wiley, Chichester (UK), 2000.
[2] Orsini, F.; Sello, G.; Sisti, M. Curr. Med. Chem. 2010, 17, 3, 264.
[3] Galezowska, J.; Gumienna-Kontecka, E. Coord. Chem. Rev. 2012, 256, 105.
[4] Wang, L.; Shen, Q.; Lu, L. Chin. J. Chem. 2013, 31, 892.
[5] Gu, L.; Wang, R.; Huang, X.; Jin, C. Chin. J. Chem. 2012, 30, 2483.
[6] Cai, Z.; Fan, Y.; Du, G.; He, L. Chin. J. Chem. 2012, 30, 1658.
[7] Aciro, C.; Davies, S. G.; Garner, A. C.; Ishii, Y.; Key, M.; Ling, K. B.; Prasad, R. S.; Roberts, P. M.; Rodriguez-Solla, H.; O'LearySteele, C.; Russell, A. J.; Sanganee, H. J.; Savory, E. D.; Smith, A. D.; Thomson, J. E. Tetrahedron 2008, 64(39), 9320.
[8] Zapata, F.; Caballero, A.; Espinosa, A.; Tarraga, A.; Molina, P. Eur. J. Inorg. Chem. 2010(5), 697.
[9] Boulos, L. S.; Ewies, E. F.; Fahmy, A. F. M.; Mohram, M. E. Phosphorus, Sulfur Silicon Relat. Elem. 2013, 188(6), 790.
[10] Aboussafy, C. L.; Clive, D. L. J. J. Org. Chem. 2012, 77(11), 5125.
[11] El-Sayed, N. F.; Ewies, E. F.; Boulos, L. S.; Moharam, M. E. Res. J. Pharm., Biol. Chem. Sci. 2014, 5(5), 926.
[12] Ramirez-Marroquin, O. A.; Romero-Estudillo, I.; Viveros-Ceballos, J. L.; Cativiela, C.; Ordonez, M. Eur. J. Org. Chem. 2016, (2), 308.
[13] Yamashita, Y.; Nam, L. C.; Dutton, M. J.; Yoshimoto, S.; Kobayashi, S. Chem. Commun. 2015, 51(96), 17064.
[14] Burchacka, E.; Skorenski, M.; Sienczyk, M.; Oleksyszyn, J. Bioorg. Med. Chem. Lett. 2013, 23(5), 1412.
[15] Klepacz, A.; Zwierzak, A. Tetrahedron Lett. 2002, 43(6), 1079.
[16] Diel, P. J.; Maier, L. Phosphorus, Sulfur Silicon Relat. Elem. 1988, 36(1~2), 85.
[17] Osapay, G.; Szilagyi, I.; Seres, J. Tetrahedron 1987, 43(13), 2977.
[18] Maier, L. Phosphorus, Sulfur Silicon Relat. Elem. 1990, 47(3~4), 361.
[19] Nasief, N. N.; Hangauer, D. Eur. J. Med. Chem. 2015, 90, 897.
[20] Zhang, D.; Yuan, C. Y. Chem.-Eur. J. 2008, 14, 6049.
[21] Yao, Q.; Yuan, C. J. Org. Chem. 2013, 78, 6962.
[22] Kapeller, D. C.; Hammerschmidt, F. Chem.-Eur. J. 2009, 15(23), 5729.
[23] Zon, J. Pol. J. Chem. 1979, 53(2), 541.
[24] Cullis, P. M.; Harger, M. J. P. J. Chem. Soc., Perkin Trans. 22002, 1538.
[25] Hirschmann, R.; Yager, K. M.; Taylor, C. M.; Witherington, J.; Sprengeler, P. A.; Phillips, B. W.; Moore, W.; Smith, A. B., Ⅲ J J. Am. Chem. Soc. 1997, 119(35), 8177.
/
| 〈 |
|
〉 |