

二茂铁苯甲酸香豆素类化合物的合成与性质研究
收稿日期: 2017-01-10
修回日期: 2017-04-07
网络出版日期: 2017-04-21
基金资助
国家自然科学基金(No.21171149)资助项目.
Synthesis and Properties of 4-Ferrocenyl-carboxybenzenecoumarin Derivatives
Received date: 2017-01-10
Revised date: 2017-04-07
Online published: 2017-04-21
Supported by
Project supported by the National Natural Science Foundation of China (No. 21171149).
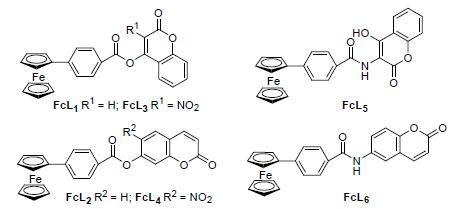
以二茂铁苯甲酸、香豆素为原料,经硝化、硝基还原和缩合等反应,设计、合成了6个新型的二茂铁苯甲酸香豆素化合物.采用红外、元素分析和核磁共振对化合物进行表征.用X射线单晶衍射测定了4-二茂铁苯甲酸(香豆素-4-基)酯(FcL1)的晶体结构.研究了这6种化合物的电化学性质和生物活性.电化学研究表明这6种物质在电极表面均可发生可逆的单电子氧化还原反应,且反应受扩散控制.生物活性测试结果表明化合物的修饰不利于杀菌活性的改善,仅有利于抗肿瘤活性的提高.其中这6种化合物对新月弯孢霉菌和玉米禾谷镰刀菌都具有较好的杀菌活性,化合物FcL1和4-二茂铁苯甲酸(香豆素-7-基)酯(FcL2)对人食管癌细胞9706 (EC-9706)的IC50分别可达到2.10和1.25 μmol/L.
李标 , 刘秋霞 , 周元清 , 贾赵栋 , 朱曼毓 , 徐琰 , 宋毛平 . 二茂铁苯甲酸香豆素类化合物的合成与性质研究[J]. 有机化学, 2017 , 37(8) : 2008 -2014 . DOI: 10.6023/cjoc201611036
Six novel ferrocenyl-carboxybenzene-coumarin derivatives were synthesized by 4-ferrocenylbenzoic and coumarin as raw materials through nitration, reduction reaction and condensation. The structures of compounds were characterized by IR, 1H NMR, 13C NMR and elemental analysis. The crystal structure of 4-ferrocene benzoic acid(coumarin-4-yl) ester (FcL1) was determined by X-ray diffraction analysis. The electrochemical research showed that the redox reaction on the surface of electrode was reversible with single electron and controlled by diffusion. Biological activity test results showed that the compound modified not conducive to the improvement of the bactericidal activity, only beneficial to the improvement of the antitumor activity. All the six compounds showed good inhibition against Curvularia lunata and Fusarium graminearum. In addition, FcL1 and 4-ferrocene benzoic acid (coumarin-7-yl) ester (FcL2) exhibited significant activities and selectivities against Esophageal carcinoma cell (IC50 value=2.10 and 1.25 μmol/L, respectively) in the anticancer activity test.

Key words: ferrocene; coumarin; electrochemical research; biological activity
[1] Wang, D.-L.; Yang, F.-F.; Liu, Z.; Dong, Z.; Zhao, W. Chin. J. Org. Chem. 2014, 34, 204(in Chinese). (王道林, 杨菲菲, 刘忠, 董哲, 赵伟, 有机化学, 2014, 34, 204.)
[2] Yang, S.-P.; Han, L.-J.; Pan, Y.; Wang, D.-Q.; Wang, N.-N.; Wang, T. Chem. J. Chin. Univ. 2013, 34, 364(in Chinese). (杨树平, 韩立军, 潘燕, 王大奇, 王南南, 王婷, 高等学校化学学报, 2013, 34, 364.)
[3] Shi, Y.; Zhou, C.-H.; Zhou, X.-D.; Geng, R.-X.; Ji, Q.-G. Acta. Pharm. Sin. 2011, 46, 798(in Chinese). (时园, 周成合, 周向东, 耿蓉霞, 吉庆刚, 药学学报, 2011, 46, 798.)
[4] Wang, D.; Wei, Y.; Hao, S.-H. Chin. J. Org. Chem. 2015, 35, 1691(in Chinese) (王栋, 魏艳, 郝双红, 有机化学, 2015, 35, 1691.)
[5] Liu, M.-M.; Chen, X.-Y.; Huang, Y.-Q.; Feng, P.; Guo, Y.-L.; Yang, G.; Chen, Y. J. Med. Chem. 2014, 57, 9343.
[6] Yeggoni, D. P.; Gokara, M.; Manidhar, D. M.; Rachamallu, A.; Nakka, S.; Reddy, C. S.; Subramanyam, R. Mol. Pharm. 2014, 11, 1117.
[7] Figueroa-Guiñez, R.; Matos, M. J.; Vazquez-Rodriguez, S.; Santana, L.; Uriarte, E.; Borges, F.; Olea-Azar, C.; Maya, J. D. Curr. Top. Med. Chem. 2015, 15, 850.
[8] Lei, Z.-L.; Hou, W.-C.; Luo, Y.-P. Chem. Reag. 2016, 38, 157(in Chinese). (雷震霖, 侯文成, 骆焱平, 化学试剂, 2016, 38, 157.)
[9] Wang, A.-L.; Tao, B.; Ai, C.-Z.; Zheng, X.-F. Chin. J. Org. Chem. 2015, 35, 843(in Chinese). (王爱玲, 陶波, 艾纯芝, 郑学仿, 有机化学, 2015, 35, 843.)
[10] Liu, M.; Liu, Y.; Liu, A.-L.; Zhang, D.-K.; Chen, M.-G.; Wu, C.-C.; Hua, X.-W.; Zhou, S.; Li, Z.-M. Chin. J. Org. Chem. 2016, 36, 1653(in Chinese). (刘明, 刘阳, 刘艾林, 张冬凯, 陈明桂, 吴长春, 华学文, 周莎, 李正名, 有机化学, 2016, 36, 1653.)
[11] Chen, H.; Zhou, L.-K.; Li, S.; Yao, Y.-C.; Gu, Y.-J.; Li, C.-X.; Li, N.; Meng, M.; Li, X.-L. Chin. J. Org. Chem. 2013, 33, 164(in Chinese). (陈华, 周利凯, 李帅, 姚玉超, 谷云景, 李春晓, 李娜, 孟明, 李小六, 有机化学, 2013, 33, 164.)
[12] Li, L.-H.; Chen, L.; Xia, Y.-F. J. China. Pharm. Univ. 2013, 44, 374(in Chinese). (李林虎, 陈莉, 夏玉凤, 中国药科大学学报, 2013, 44, 374.)
[13] Yang, S.-P.; Han, L.-J.; Pan, Y.; Wang, D.-Q.; Zhou, Y.-N.; Zhang, F. Sci. China, Ser. B 2013, 43, 858(in Chinese). (杨树平, 韩立军, 潘燕, 王大奇, 周亚男, 张凡, 中国科学B辑:化学, 2013, 43, 858.)
[14] Wang, D.-W.; Yu, X.; Yao, W.; Hu, W.-K.; Ge, C.-Y.; Shi, X.-D. Chem.-Eur. J. 2016, 22, 5543.
[15] Zhuang, H.; Zeng, R.-S.; Zou, J.-P. Chin. J. Chem. 2016, 34, 368.
[16] Wang, D.-W.; Ge, B.-Y.; Li, L.; Shan, J.; Ding, Y,-Q. J. Org. Chem. 2014, 79, 8607.
[17] Li, J.; Zeng, Y.; Zhang, X.-H; Yu, T.-J; Chen, J.-P.; Li, Y. Acta Chim. Sinica 2015, 73, 826(in Chinese). (李婧, 曾毅, 张小辉, 于天君, 陈金平, 李嫕, 化学学报, 2015, 73, 826.)
[18] Wang, D.-W.; Yu, X.-L.; Ge, B.-Y.; Miao, H.-Y.; Ding, Y.-Q. Chin. J. Org. Chem. 2015, 35, 676(in Chinese). (王大伟, 余晓丽, 葛冰洋, 苗红艳, 丁玉强, 有机化学, 2015, 35, 676.)
[19] Fan, W.; Li, M.; Hong, C.-Y.; Pan, C.-Y.; Acta Chim. Sinica 2015, 73, 330(in Chinese). (范溦, 李敏, 洪春雁, 潘才元, 化学学报, 2015, 73, 330.)
[20] Gacche, R. N.; Jadhav, S. G. J. Exp. Clin. Med. 2012, 4, 165.
[21] Yang, J.; Liu, G.-Y.; Dai, F.; Cao, X.-Y.; Kang, Y.-F.; Hu, L.-M.; Tang, J.-J.; Li, X.-Z.; Li, Y.; Jin, X.-L.; Zhou, B. Bioorg. Med. Chem. Lett. 2011, 21, 6420.
[22] Serra, S.; Chicca, A.; Delogu, G.; Vázquez-Rodríguez, S.; Santana, L.; Uriarte, E.; Casu, L.; Gertsch, J. Bioorg. Med. Chem. Lett. 2012, 22, 5791.
[23] Zhang, W.-H.; Jiang, M.-G. Chin. J. Org. Chem. 2010, 30, 254(in Chinese). (章维华, 蒋木庚, 有机化学, 2010, 30, 254).
[24] Vázquez, R.; Riveiro, M, E.; Vermeulen, M.; Alonso, E.; Mondillo, C.; Facorro, G.; Piehl, L.; Gómez, N.; Moglioni, A.; Fernández, N.; Baldi, A.; Shayo, C.; Davio, C. Bioorg. Med. Chem. 2012, 20, 5537.
[25] Kempen, I.; Papapostolou, D.; Thierry, N.; Pochet, L.; Counerotte, S.; Masereel, B.; Foidart, J. F.; Reboud-Ravaux, M.; Noel, A.; Pirotte, B. Brit. J. Cancer 2003, 88, 1111.
[26] Al-Amiery, A. A.; Al-Bayati, R. I. H.; Saour, K. Y.; Radi, M. F. Res. Chem. Intermed. 2012, 38, 559.
[27] Wu, K.-L.; Zhang, W.-B.; Zhou, D.; Xu, Y. Chin. J. Org. Chem. 2014, 34, 1201(in Chinese). (吴孔丽, 张吾斌, 周丹, 徐琰, 有机化学, 2014, 34, 1201.)
[28] Wang, D.-W.; Yu, X.-L.; Xu, X.; Ge, B.-Y.; Wang, X.-L.; Zhang, Y.-X. Chem.-Eur. J. 2016, 22, 8663.
[29] Pan, C.-X.; Wang, Z.-C.; Su, G.-F.; Zong, X.; Xue, W.-B.; Tan, J.-K. Chin. J. Org. Chem. 2012, 32, 742(in Chinese). (潘成学, 王忠长, 苏桂发, 宗玺, 薛文彬, 覃江克, 有机化学, 2012, 32, 742.)
[30] Liu, W.-H.; Wang, S.-B.; Chang, J.-X.; Liu, Y. Acta Pharm. Sin. 2014, 49, 217(in Chinese). (刘文虎, 王仕宝, 常晋霞, 刘毅, 药学学报, 2014, 49, 217.)
[31] (a) Tsutomu, I.; Hiroyuki, S.; Akira, O. JP 2004066547, 2004,[Chem. Abstr. 2004, 140, 225859].
(b) Rao, H. S.; Sivakumar, S. J. Org. Chem. 2006, 71, 8715.
(c) Verma, R. K.; Verma, G. K.; Shukla, G.; Singh, M. S. RSC Adv. 2012, 2, 2413.
(d) Wang, W.-F.; Fan, J.; Luo, C.-Z.; Yuan, Y.-F.; Zhang, Y.-F. ARKIVOC 2011, 44, 105.
[32] CCDC 1487759 contains the supplementary crystallographic data for coupound FcL1. These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.
[33] Xu, Y.; Zhu, L.-M.; Ran, C.-L.; Wang, H.-X.; Fan, Y.-T. Chin. J. Inorg. Chem. 2007, 23, 589(in Chinese). (徐琰, 朱丽敏, 冉春玲, 王海先, 樊耀亭, 无机化学学报, 2007, 23, 589.)
[34] Chen, Y.-W; Wan, Y.-Y.; Liu, Q.-X.; Liu, J.-B.; Xiong, L.-X.; Yu, S.-J.; Li, Z.-M. Chin. J. Org. Chem. 2015, 35, 882(in Chinese). (陈有为, 万莹莹, 刘巧霞, 刘敬波, 熊丽霞, 于淑晶, 李正名, 有机化学, 2015, 35, 882.)
[35] Jiang, W.-T.; Hu, F.-Z.; Gu, H.; Liu, C.; Wei, N.-X.; Wan, L.; Ren, S.-Z.; Wang, J.-T.; Xu, F.-B. Chin. J. Org. Chem. 2014, 34, 774(in Chinese). (姜文涛, 胡方中, 顾翰, 刘传, 魏乃翔, 万蕾, 任士钊, 王俊婷, 徐凤波, 有机化学, 2014, 34, 774.)
[36] Zhang, H. X.; He, Q. Y. J. Pharm. Res. 2016, 35, 63(in Chinese). (张会鲜, 何琪杨, 药学研究, 2016, 35, 63.)
[37] Wang, C.; Jiang, R.-S.; Feng, F.; Fu, G.-L. Chem. Reag. 2008, 30, 935(in Chinese). (王诚, 江润生, 冯锋, 付国良, 化学试剂, 2008, 30, 935.)
[38] (a) Robertson, G. R.; Evans, R. A. J. Org. Chem. 1940, 5, 142.
(b) Li, M.-H.; Wang, J.; Fang, S.-D.; He, Z.-L. Chin. J. Spectrosc. Lab. 2001, 18, 63(in Chinese). (李明慧, 王井, 方世东, 何钟林, 光谱实验室, 2001, 18, 63.)
(c) Wang, D.-T; Wang, Y.-P. Mater. Sci. Technol. 2005, 13, 662(in Chinese). (王东田, 王郁萍, 材料科学与工艺, 2005, 13, 662.)
/
| 〈 |
|
〉 |