

钯/碳催化三唑导向C—H键活化芳基化
收稿日期: 2016-12-22
修回日期: 2017-04-03
网络出版日期: 2017-04-21
基金资助
国家自然科学基金(No.20702051)、浙江省自然科学基金(No.LY13B020017)、浙江省科技重点创新团队(No.2010R50018)资助项目
Pd/C Catalyzed C—H Arylation with Click-Triazoles as Removable Directing Group
Received date: 2016-12-22
Revised date: 2017-04-03
Online published: 2017-04-21
Supported by
Project supported by the National Natural Science Foundation of China (No. 20702051), the Natural Science Foundation of Zhejiang Province (No. LY13B020017) and the Key Innovation Team of Science and Technology in Zhejiang Province (No. 2010R50018).
谢晓强 , 邢运哲 , 张国富 , 丁成荣 . 钯/碳催化三唑导向C—H键活化芳基化[J]. 有机化学, 2017 , 37(8) : 2124 -2130 . DOI: 10.6023/cjoc201612054
A novel protocol for Pd/C catalyzed C(sp2)—H arylation of benzamides with good functional group compatibility under silver-free conditions by using click-triazoles as a removable directing group has been first developed.
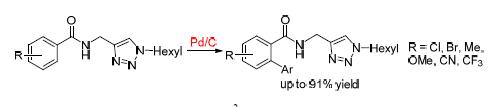
Key words: Pd/C; click-triazoles; arylation; C—H bond activation
[1] (a) Lin, G.-Q.; Cheng, Y.-Q.; Cheng, X.-C.; Li, Y.-M. Chiral Synthesis-Principles and Application of Asymmetric Reaction, Science Press, Beijing, 2000(in Chinese). (林国强, 陈耀全, 陈新滋, 李月明, 手性合成-不对称反应及其应用, 科学出版社, 北京, 2000.)
(b) De Mejiere, A.; Diederich, F. Metal-Catalyzed Cross-Coupling Reactions, Wiley-VCH, Weinheim, 2004.
(c) Liu, C.; Zhang, H.; Shi, W.; Lei, A.-W. Chem. Rev. 2011, 11, 1780.
(d) Li, W.-Y.; Zhao, D.-M.; Xiong, X.-Q.; Ma, Q.-Q.; Cheng, M.-A. Chin. J. Org. Chem. 2011, 31, 784(in Chinese). (李文燕, 赵冬梅, 熊绪琼, 马倩倩, 程卯生, 有机化学, 2011, 31, 784.)
[2] (a) Miyaura, N.; Suzuki, A. J. Chem. Soc., Chem. Commun. 1979, 866.
(b) Miyaura, N.; Yamada, K.; Suzuki, A. Tetrahedron Lett. 1979, 36, 3437.
(c) Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457.
(d) Stanforth, S. P. Tetrahedron 1998, 54, 263.
(e) Suzuki, A. Chem. Commun. 2005, 38, 4759.
[3] (a) Milstein, D.; Stille, J. K. J. Am. Chem. Soc. 1978, 100, 3636.
(b) Milstein, D.; Stille, J. K. J. Am. Chem. Soc. 1979, 101, 4981.
(c) Milstein, D.; Stille, J. K. J. Am. Chem. Soc. 1979, 101, 4992.
[4] (a) Baba, S.; Negishi, E. J. Am. Chem. Soc. 1976, 98, 6729.
(b) Negishi, E.; Baba, S. J. Chem. Soc., Chem. Commun. 1976, 596.
[5] For selected reviews on C-H activations, see:(a) Li, B.-J.; Yang, S.-D.; Shi, Z.-J. Synlett 2008, 949.
(b) Daugulis, O.; Do, H.-Q.; Shabashow, D. Acc. Chem. Rev. 2009, 42, 1074.
(c) Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094.
(d) Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147.
(e) Song, G.; Wang, F.; Li, X. Chem. Soc. Rev. 2012, 41, 3651.
(f) Li, D.; He, C.; Cai, H.; Wang, G. Chin. J. Org. Chem. 2013, 33, 203(in Chinese). (李丹丹, 何程林, 蔡海婷, 王官武, 有机化学, 2013, 33, 203.)
(g) Kuhl, N.; Schroder, N.; Glorius, F. Adv. Synth. Catal. 2014, 356, 1443.
(h) Zhang, B.; Guan, H.-X; Liu, B.; Shi, B.-F. Chin. J. Org. Chem. 2014, 34, 1487(in Chinese). (张博, 管晗曦, 刘斌, 史炳锋, 有机化学, 2014, 34, 1487.)
(i) Girard, S. A.; Knauber, T.; Li, C.-J. Angew. Chem., Int. Ed. 2014, 53, 74.
(j) Song, G.; Li, X. Acc. Chem. Res. 2015, 48, 1007.
(k) Ye, B.; Cramer, N. Acc. Chem. Res. 2015, 48, 1308.
(l) Yang, L.; Huang, H. Chem. Rev. 2015, 115, 3468.
(m) Gang, F.-L.; Xu, G.-L.; Dong, T.-S.; Yang, L.; Du, Z.-Y. Chin. J. Org. Chem. 2015, 35, 1428(in Chinese). (刚芳莉, 徐光利, 董涛生, 杨丽, 杜正银, 有机化学, 2015, 35, 1428.)
(n) Yuan, Y.-Z.; Song, S.; Jiao, N. Acta Chim. Sinica 2015, 73, 1231(in Chinese). (袁逸之, 宋颂, 焦宁, 化学学报, 2015, 73, 1231.)
(o) Shang, X.-J.; Liu, Z.-Q. Acta Chim. Sinica 2015, 73, 1275(in Chinese). (尚筱洁, 柳忠全, 化学学报, 2015, 73, 1275.)
(p) Liao, G.; Shi, B.-F. Acta Chim. Sinica 2015, 73, 1283(in Chinese). (廖港, 史炳峰, 化学学报, 2015, 73, 1283.)
(q) Wang, L.; Li, Z.; Qu, X.; Peng, W.-M. Chin. J. Chem. 2015, 33, 1015.
(r) Zhu, Q.; Wang, L.; Xia, C.-G.; Liu, C. Chin. J. Org. Chem. 2016, 36, 2813(in Chinese). (朱庆, 王露, 夏春谷, 刘超, 有机化学, 2016, 36, 2813.)
(s) Luo, F.-H.; Long, Y.; Li, Z.-K.; Zhou, X.-G. Acta Chim. Sinica 2016, 74, 805(in Chinese). (罗飞华, 龙洋, 李正凯, 周向葛, 化学学报, 2016, 74, 805.)
[6] (a) Brase, S.; Waegell, B.; Meijere, A. Synthesis 1998, 148.
(b) Deng, W.; Liu, L.; Guo, Q.-X. Chin. J. Org. Chem. 2004, 24, 150(in Chinese). (邓维, 刘磊, 郭庆祥, 有机化学, 2004, 24, 150.)
(c) Wu, X.-H.; Yang, Z.-C.; Qin, W.; Jiang, Z.-H. Chin. J. Org. Chem. 2006, 26, 260(in Chinese). (吴晓宏, 杨占成, 秦伟, 姜兆华, 有机化学, 2006, 26, 260.)
(d) Tang, D. D.; Collins, K. D.; Glorius, K. J. Am. Chem. Soc. 2013, 135, 7450.
(e) Shu, Z.; Li, W.; Wang, B.-Q. Chem. Cat. Chem. 2015, 7, 605.
(f) Collins, K. D.; Honeker, R.; Vasquez-Cespedes, S.; Tang, D. D.; Glorius, K. Chem. Sci. 2015, 6, 1816.
[7] Zhang, G.-F.; Xie, X.-Q.; Zhu, J.-F.; Li, S.-S.; Ding, C.-R.; Ding, P. Org. Biomol. Chem. 2015, 13, 5444.
[8] (a) Ye, X.-H.; He, Z.-R.; Ahmed, T.; Weise, K.; Akhmedov, N. J.; Petersena, J. L. Shi, X.-D. Chem. Sci. 2013, 4, 3712.
(b) Ye, X.-H.; Shi, X.-D. Org. Lett. 2014, 16, 4448.
(c) Al Mamari, H.; Diers, E.; Ackermann, L. Chem.-Eur. J. 2014, 20, 9739.
(d) Gu, Q.; AlMamari, H.; Graczyk, K.; Diers, E.; Ackermann, L. Angew. Chem., Int. Ed. 2014, 53, 3868.
(e) Tirler, C.; Ackermann, L. Tetrahedron 2015, 71, 4543.
(f) Ye, X.-H.; Xu, C.; Wojtas, L.; Akhmedov, N. J.; Chen, H.; Shi, X.-D. Org. Lett. 2016, 18, 2970.
(g) Ye, X.-H.; Zhang, Y.-W.; He, Y.; Shi, X.-D. Tetrahedron 2016, 72, 2756.
(h) Cera, G.; Haven, T.; Ackermann, L. Angew. Chem., Int. Ed. 2016, 55, 1484.
(i) Santrac, D.; Cella, S.; Wang, L.; Ackermann, L. Eur. J. Org. Chem. 2016, 32, 5429.
[9] Bielawski, M.; Aili, D.; Olofsson, B. J. Org. Chem. 2008, 73, 4602.
/
| 〈 |
|
〉 |