

钯催化卤代烷烃参与的自由基型转化反应
收稿日期: 2017-02-28
修回日期: 2017-05-05
网络出版日期: 2017-05-17
基金资助
国家重点基础研究发展计划(973计划,No.2015CB856600)资助项目.
Palladium-Catalyzed Radical-Type Transformations of Alkyl Halides
Received date: 2017-02-28
Revised date: 2017-05-05
Online published: 2017-05-17
Supported by
Project supported by the National Program on Key Basic Research Project of China (973 Program, No. 2015CB856600).
周文俊 , 张逸寒 , 曹光梅 , 刘惠东 , 余达刚 . 钯催化卤代烷烃参与的自由基型转化反应[J]. 有机化学, 2017 , 37(6) : 1322 -1337 . DOI: 10.6023/cjoc201702051
Palladium-catalyzed cross-coupling reactions have been developed for decades as useful methods in organic synthesis. Compared to aryl and alkenyl halides, alkyl halides are more challenging to be applied in cross-coupling reactions. This mainly arises from the difficulty in oxidative addition of alkyl halides to palladium catalyst, sluggish reductive elimination and competitive side reactions, such as β-H elimination and protonation, of the resulting alkylpalladium intermediates. These challenges have partly been overcome with the significant development of novel palladium catalysis involving single election transfer. A variety of cross couplings of alkyl halides have been developed. In this review the recent palladium-catalyzed radical alkylation using alkyl halides with the order of different types of coupling partners is summarized.
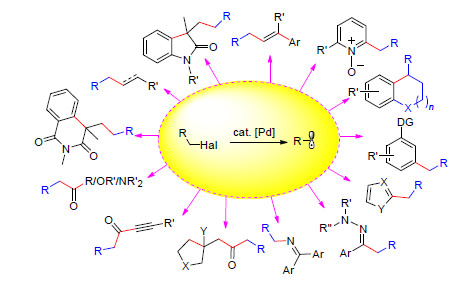
Key words: palladium catalysis; alkyl halide; free radical
[1] (a) Kochi, J. K.; Tamura, M. J. Am. Chem. Soc. 1971, 93, 1483.
(b) Tamura, M.; Kochi, J. K. J. Organomet. Chem. 1972, 42, 205.
(c) Ishiyama, T.; Abe, S.; Miyaura, N.; Suzuki, A. Chem. Lett. 1992, 21, 691.
(d) Devasagayaraj, A.; Stüdemann, T.; Knochel, P. Angew. Chem., Int. Ed. 1996, 34, 2723.
[2] (a) Ryu, I. Chem. Soc. Rev. 2001, 30, 16.
(b) Frisch, A. C.; Beller, M. Angew. Chem., Int. Ed. 2005, 44, 674.
(c) Ackermann, L. Chem. Commun. 2010, 46, 4866.
(d) Sumino, S.; Fusano, A.; Fukuyama, T.; Ryu, I. Acc. Chem. Res. 2014, 47, 1563.
(e) Sebren, L. J.; Devery, J. J.; Stephenson, C. R. J. ACS Catal. 2014, 4, 703.
(f) Zhang, W.; Dai, J.; Xu, H. Chin. J. Org. Chem. 2015, 35, 1820 (in Chinese).(张文曼, 戴建军, 许华建, 有机化学, 2015, 35, 1820.)
[3] Kambe, N.; Iwasaki, T.; Terao, J. Chem. Soc. Rev. 2011, 40, 4937.
[4] Ishihara, T.; Kuroboshi, M.; Okada, Y. Chem. Lett. 1986, 15, 1895.
[5] Curran, D. P.; Chang, C.-T. Tetrahedron Lett. 1990, 31, 933.
[6] Yi, P.; Zhang, Z.; Hu, H. Synth. Commun. 1992, 22, 2019.
[7] Surapanich, N.; Kuhakarn, C.; Pohmakotr, M.; Reutrakul, V. Eur. J. Org. Chem. 2012, 2012, 5943.
[8] Zou, Y.; Zhou, J. Chem. Commun. 2014, 50, 3725.
[9] Bissember, A. C.; Levina, A.; Fu, G. C. J. Am. Chem. Soc. 2012, 134, 14232.
[10] McMahon, C. M.; Alexanian, E. J. Angew. Chem., Int. Ed. 2014, 53, 5974.
[11] Bloome, K. S.; McMahen, R. L.; Alexanian, E. J. J. Am. Chem. Soc. 2011, 133, 20146.
[12] (a) Hidai, M.; Kokura, M.; Uchida, Y. J. Organomet. Chem. 1973, 52, 431.
(b) Ozawa, F.; Sugimoto, T.; Yuasa, Y.; Santra, M.; Yamamoto, T.; Yamamoto, A. Organometallics 1984, 3, 683.
(c) Cavinato, G.; Toniolo, L.; Vavasori, A. J. Mol. Catal. A: Chem. 2004, 219, 233.
[13] Dong, X.; Han, Y.; Yan, F.; Liu, Q.; Wang, P.; Chen, K.; Li, Y.; Zhao, Z.; Dong, Y.; Liu, H. Org. Lett. 2016, 18, 3774.
[14] Sumino, S.; Ryu, I. Org. Lett. 2016, 18, 52.
[15] Liu, H.; Qiao, Z.; Jiang, X. Org. Biomol. Chem. 2012, 10, 7274.
[16] Fan, J.-H.; Wei, W.-T.; Zhou, M.-B.; Song, R.-J.; Li, J.-H. Angew. Chem., Int. Ed. 2014, 53, 6650.
[17] Liu, Q.; Chen, C.; Tong, X. Tetrahedron Lett. 2015, 56, 4483.
[18] Wang, H.; Guo, L.-N.; Duan, X.-H. J. Org. Chem. 2016, 81, 860.
[19] Xia, X.-F.; Zhu, S.-L.; Li, Y.; Wang, H. RSC Adv. 2016, 6, 51703.
[20] Fruchey, E. R.; Monks, B. M.; Patterson, A. M.; Cook, S. P. Org. Lett. 2013, 15, 4362.
[21] Monks, B. M.; Cook, S. P. Angew. Chem., Int. Ed. 2013, 52, 14214.
[22] Li, Z.; García-Domínguez, A.; Nevado, C. J. Am. Chem. Soc. 2015, 137, 11610.
[23] He, Y.-T.; Wang, Q.; Li, L.-H.; Liu, X.-Y.; Xu, P.-F.; Liang, Y.-M. Org. Lett. 2015, 17, 5188.
[24] Domański, S.; Chaladaj, W. ACS Catal. 2016, 6, 3452.
[25] Wang, Q.; He, Y.-T.; Zhao, J.-H.; Qiu, Y.-F.; Zheng, L.; Hu, J.-Y.; Yang, Y.-C.; Liu, X.-Y.; Liang, Y.-M. Org. Lett. 2016, 18, 2664.
[26] Xiao, B.; Liu, Z.-J.; Liu, L.; Fu, Y. J. Am. Chem. Soc. 2013, 135, 616.
[27] Wu, X.; See, J. W. T.; Xu, K.; Hirao, H.; Roger, J.; Hierso, J.-C.; Zhou, J. Angew. Chem., Int. Ed. 2014, 53, 13573.
[28] Shao, C.; Shi, G.; Zhang, Y.; Pan, S.; Guan, X. Org. Lett. 2015, 17, 2652.
[29] Venning, A. R. O.; Bohan, P. T.; Alexanian, E. J. J. Am. Chem. Soc. 2015, 137, 3731.
[30] Li, Z.-Y.; Li, L.; Li, Q.-L.; Jing, K.; Xu, H.; Wang, G.-W. Chem. Eur. J. 2017, 23, 3285.
[31] Liu, Q.; Dong, X.; Li, J.; Xiao, J.; Dong, Y.; Liu, H. ACS Catal. 2015, 5, 6111.
[32] Tsuji, J.; Sato, K.; Nagashima, H. Tetrahedron 1985, 41, 5003.
[33] Urata, H.; Ishii, Y.; Fuchikami, T. Tetrahedron Lett. 1989, 30, 4407.
[34] Ishiyama, T.; Miyaura, N.; Suzuki, A. Tetrahedron Lett. 1991, 32, 6923.
[35] Ishiyama, T.; Murata, M.; Suzuki, A.; Miyaura, N. J. Chem. Soc., Chem. Commun. 1995, 295.
[36] Ryu, I.; Kreimerman, S.; Araki, F.; Nishitani, S.; Oderaotoshi, Y.; Minakata, S.; Komatsu, M. J. Am. Chem. Soc. 2002, 124, 3812.
[37] Fukuyama, T.; Nishitani, S.; Inouye, T.; Morimoto, K.; Ryu, I. Org. Lett. 2006, 8, 1383.
[38] Bloome, K. S.; Alexanian, E. J. J. Am. Chem. Soc. 2010, 132, 12823.
[39] Fusano, A.; Fukuyama, T.; Nishitani, S.; Inouye, T.; Ryu, I. Org. Lett. 2010, 12, 2410.
[40] (a) Fusano, A.; Sumino, S.; Fukuyama, T.; Ryu, I. Org. Lett. 2011, 13, 2114.
(b) Fusano, A.; Sumino, S.; Nishitani, S.; Inouye, T.; Morimoto, K.; Fukuyama, T.; Ryu, I. Chem. Eur. J. 2012, 18, 9415.
[41] (a) Sumino, S.; Fusano, A.; Fukuyama, T.; Ryu, I. Synlett 2012, 23, 1331.
(b) Sumino, S.; Ui, T.; Ryu, I. Org. Lett. 2013, 15, 3142.
(c) Sumino, S.; Ui, T.; Hamada, Y.; Fukuyama, T.; Ryu, I. Org. Lett. 2015, 17, 4952.
(d) Sumino, S.; Ui, T.; Ryu, I. Org. Chem. Front. 2015, 2, 1085.
[42] Zhao, H.-Y.; Feng, Z.; Luo, Z.; Zhang, X. Angew. Chem., Int. Ed. 2016, 55, 10401.
[43] Sargent, B. T.; Alexanian, E. J. J. Am. Chem. Soc. 2016, 138, 7520.
[44] Peacock, D. M.; Roos, C. B.; Hartwig, J. F. ACS Cent. Sci. 2016, 2, 647.
[45] Prieto, A.; Melot, R.; Bouyssi, D.; Monteiro, N. Angew. Chem., Int. Ed. 2016, 55, 1885.
/
| 〈 |
|
〉 |