

具有靛红结构的吡唑衍生物的合成及其诱导非小细胞肺癌A549细胞凋亡的研究
收稿日期: 2017-04-06
修回日期: 2017-05-05
网络出版日期: 2017-05-17
基金资助
贵州省科技厅(Nos.[2014]7565,[2014]7557,[2015]6010)、遵义医学院博士科研启动基金(No.F-631)、国家级大学生创新创业(No.201510661009)、遵义医学院大学生创新创业(Nos.[2014]5811,[2014]2918)和遵义医学院学科建设(药物化学)资助项目.
Synthesis of Novel Pyrazole Derivatives Containing Isatins as Potential Apoptosis Inducer in Non-small Lung Cancer A549 Cells
Received date: 2017-04-06
Revised date: 2017-05-05
Online published: 2017-05-17
Supported by
Project supported by the Department of Science and Technology of Guizhou Province (Nos.[2014]7565,[2014]7557,[2015]6010),the Priming Scientific Research Foundation for Doctoral Program of Zunyi Medical University (No.F-631),the National Undergraduate Training Programs for Innovation and Entrepreneurship (No.201510661009),the Undergraduate Training Programs for Innovation and Entrepreneurship of Zunyi Medical University (Nos.[2014]5811,[2014]2918) and the Discipline Construction Funding (Medicinal Chemistry) of Zunyi Medical University.
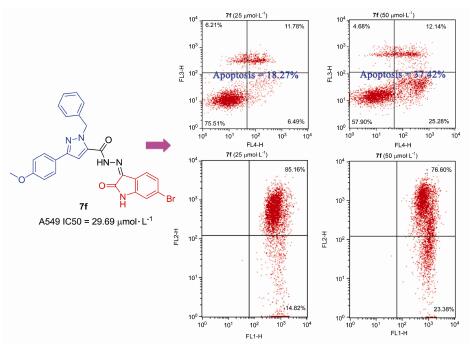
基于药效团拼合的设计原理,以4-羟基苯乙酮为原料,通过加成、环化、取代、肼解和缩合等多步反应构建了两类含有靛红结构的吡唑衍生物,共计12个目标产物,其结构经1H NMR,13C NMR和HRMS确证.采用细胞计数试剂盒-8(CCK-8)法测试目标产物对人非小细胞肺癌细胞A549的体外抑制作用,部分化合物显示出一定的抗增殖活性,其中N'-(3-亚氨基-6-氯吲哚-2-酮)-1-苯甲基-3-(4-甲氧基苯基)-1H-吡唑-5-甲酰肼(7e)和N'-(3-亚氨基-6-溴吲哚-2-酮)-1-苯甲基-3-(4-甲氧基苯基)-1H-吡唑-5-甲酰肼(7f)的IC50值分别为30.41和29.69 μmol/L.流式细胞术检测表明,化合物7f能够剂量依赖性地诱导A549细胞凋亡,并降低线粒体膜电位,但对细胞周期没有影响.上述研究证明,目标产物7f能够通过线粒体途径诱导A549细胞凋亡,从而发挥抗肿瘤活性.
张磊 , 李文赟 , 刘来 , 郑诚月 , 王杨 , 徐应淑 , 史大斌 , 聂绪强 , 国佳莹 , 朱春媛 , 王京 . 具有靛红结构的吡唑衍生物的合成及其诱导非小细胞肺癌A549细胞凋亡的研究[J]. 有机化学, 2017 , 37(7) : 1721 -1729 . DOI: 10.6023/cjoc201704006
Two kinds of novel pyrazole derivatives containing isatins, total of 12 target compounds, were prepared from 4-hydroxyacetophenone via addition, cyclization, substitution, hydrazinolysis and condensation, which were characterized by 1H NMR, 13C NMR and HRMS. The anti-neoplastic activity of target molecules was evaluated against human non-small cell lung cancer cell line A549 using cell counting Kit-8 (CCK-8) assay. The data showed that several compounds possessed potential anticancer effect. Among them, N'-(3-imino-6-chloroindole-2-one)-1-benzyl-3-(4-methoxyphenyl)-1H-pyrazole-5-carbohydrazide (7e) and N'-(3-imino-6-bromoindole-2-one)-1-benzyl-3-(4-methoxyphenyl)-1H-pyrazole-5-carbohydrazide (7f) exhibited IC50 values of 30.41 and 29.69 μmol/L, respectively. Flow cytometry showed that compound 7f could induce apoptosis of A549 cells, reduce the mitochondrial membrane potential, but have no effect on the cell cycle. Those data indicated that the anti-proliferative activity of molecule 7f was mediated by apoptosis-dependent mechanism involving the mitochondrial pathway in A549 cells.

[1] Brody, H. Nature 2014, 513, S1.
[2] Chen, W.; Zheng, R.; Baade, P. D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X. Q.; He, J. CA Cancer J. Clin. 2016, 66, 115.
[3] Chougule, M.; Patel, A. R.; Sachdeva, P.; Jackson, T.; Singh, M. Lung Cancer 2011, 71, 271.
[4] Zhang, L.; Lin, Y.; Wang, J.; Zhu, X.; Yao, Q.; Yao, Q. Chin. J. Org. Chem. 2015, 35, 497(in Chinese). (张磊, 林娅, 王京, 朱心玲, 姚秋丽, 姚其正, 有机化学, 2015, 35, 497.)
[5] Yu, L. G.; Ni, T. F.; Gao, W.; He, Y.; Wnag, Y. Y.; Cui, H. W.; Yang, C. G.; Qiu, W. W. Eur. J. Med. Chem. 2015, 90, 10.
[6] Chavan, H. V.; Bandgar, B. P.; Adsul, L. K.; Dhakane, V. D.; Bhale, P. S.; Thakare, V. N.; Masand, V. Bioorg. Med. Chem. Lett. 2013, 23, 1315.
[7] Yan, T.; Yu, S. J.; Liu, P. F.; Liu, Z.; Wang, B. L.; Xiong, L. X.; Li, Z. M. Chin. J. Chem. 2012, 30, 919.
[8] Wen, Y.; Zhang, S.; Hou, G.; Yu, Y.; Gao, J. Chin. J. Org. Chem. 2016, 36, 642(in Chinese). (温彦鹏, 张爽, 侯广峰, 于颖慧, 高金胜, 有机化学, 2016, 36, 642.)
[9] Penning, T. D.; Talley, J. J.; Bertenshaw, S. R.; Carter, J. S.; Collins, P. W.; Docter, S.; Graneto, M. J.; Lee, L. F.; Malecha, J. W.; Miyashiro, J. M.; Rogers, R. S.; Rogier, D. J.; Yu, S. S.; Anderson, G. D.; Burton, E. G.; Cogburn, J. N.; Gregory, S. A.; Koboldt, C. M.; Perkins, W. E.; Seibert, K.; Veenhuizen, A. W.; Zhang, Y. Y.; Isakson, P. C. J. Med. Chem. 1997, 40, 1347.
[10] Hou, Q. Q.; Li, Z. N.; Liu, C. L. Pesticides 2002, 41, 41(in Chinese). (侯春青, 李志念, 刘长令, 农药, 2002, 41, 41.)
[11] Hu-Lowe, D. D.; Zou, H. Y.; Grazzini, M. L.; Hallin, M. E.; Wickman, G. R.; Amundson, K.; Chen, J. H.; Rewolinski, D. A.; Yamazaki, S.; Wu, E. Y.; McTigue, M. A.; Murray, B. W.; Kania, R. S.; O'Connor, P.; Shalinsky, D. R.; Bender, S. L. Clin. Cancer Res. 2008, 14, 7272.
[12] Xia, Y.; Dong, Z. W.; Zhao, B. X.; Ge, X.; Meng, N.; Shin, D. S.; Miao, J. Y. Bioorg. Med. Chem. 2007, 15, 6893.
[13] Zheng, L. W.; Wu, L. L.; Zhao, B. X.; Dong, W. L.; Miao, J. Y. Bioorg. Med. Chem. 2009, 17, 1957.
[14] Song, L.; Su, H.; Zhao, B. X.; Liu, W. Y.; Zheng, L. W.; Miao, J. Y. Bioorg. Med. Chem. 2009, 17, 7085.
[15] Zhang, J. F.; Li, M.; Miao, J. Y.; Zhao, B. X. Eur. J. Med. Chem. 2014, 83, 516.
[16] Vujasinovi?, I.; Paravi?-Radi?evi?, A.; Mlinari?-Majerski, K.; Brajša, K.; Bertoša, B. Bioorg. Med. Chem. 2012, 20, 2101.
[17] Wang, J.; Zhang, L.; Yao, Q. Z. Chin. J. Synth. Chem. 2014, 22, 730(in Chinese). (王京, 张磊, 姚其正, 合成化学, 2014, 22, 730.)
[18] Zhang, L.; Wang, J.; Zhu, X. L.; Liu, Y.; Yao, Q. Z. Chem. Reag. 2015, 37, 25(in Chinese). (张磊, 王京, 朱心玲, 林娅, 姚其正, 化学试剂, 2015, 37, 25.)
[19] Zhang, L.; Wang, J.; Lin, Y.; Zhu, X. L.; Yao, Q. Z. Chin. J. Synth. Chem. 2015, 23, 499(in Chinese). (张磊, 王京, 林娅, 朱心玲, 姚其正, 合成化学, 2015, 23, 499.)
[20] Welsch, M. E.; Snyder, S. A.; Stockwell, B. R. Curr. Opin. Chem. Biol. 2010, 14, 347.
[21] Vine, K. L.; Matesic, L.; Locke, J. M.; Ranson, M.; Skropeta, D. Anti-Cancer Agents Med. Chem. 2009, 9, 397.
[22] Zhang, Y.; Lv, M.; Zhang, Y.; Chen, L.; Wang, W.; Li, B. Chin. J. Org. Chem. 2017, 37, 1789(in Chinese). (张颖, 吕梦娇, 张娅玲, 陈丽, 王伟, 李宝林, 有机化学, 2017, 37, 1789.)
[23] Hsuan, S. L.; Chang, S. C.; Wang, S. Y.; Liao, T. L.; Jong, T. T.; Chien, M. S.; Lee, W. C.; Chen, S. S.; Liao, J. W. J. Ethnopharmacol. 2009, 123, 61.
[24] Sun, L.; Liang, C.; Shirazian, S.; Zhou, Y.; Miller, T.; Cui, J.; Fukuda, J. Y.; Chu, J. Y.; Nematalla, A.; Wang, X.; Chen, H.; Sistla, A.; Luu, T. C.; Tang, F.; Wei, J.; Tang, C. J. Med. Chem. 2003, 46, 1116.
[25] Zhang, L.; Chne, F.; Wang, J.; Chen, Y.; Zhang, Z.; Lin, Y.; Zhu, X. RSC Adv. 2015, 5, 97816.
[26] Nagarsenkar, A.; Guntuku, L.; Guggilapu, S. D.; Bai K, D.; Gannoju, S.; Naidu, V. G. M.; Bathini, N. B. Eur. J. Med. Chem. 2016, 124, 782.
[27] Eldehna, W. M.; Altoukhy, A.; Mahrous, H.; Abdel-Azizc, H. A. Eur. J. Med. Chem. 2015, 90, 684.
[28] Evdokimova, N. M.; Magedov, I. V.; McBrayer, D.; Kornienko, A. Bioorg. Med. Chem. Lett. 2016, 26, 1558.
[29] Meunier, B. Acc. Chem. Res. 2007, 41, 69.
[30] Shaveta, S. M.; Singh, P. Eur. J. Med. Chem. 2016, 124, 500.
[31] Ly, J. D.; Grubb, D. R.; Lawen, A. Apoptosis 2003, 8, 115.
[32] Cao, S.; Xu, X.; Liao, J.; Ma, L.; Ding, P.; Lin, H.; Gao, M. CN 104311470, 2015[Chem. Abstr. 2015, 162, 272777].
/
| 〈 |
|
〉 |