

金属催化的共轭二烯的1,2-双官能化反应的研究进展
收稿日期: 2017-04-18
修回日期: 2017-05-22
网络出版日期: 2017-05-25
基金资助
国家自然科学基金(Nos.21232004,21672142)、上海市优秀学科带头人(No.14XD1402300)和上海市科委基础研究基金(No.15JC1402200)资助项目.
Recent Advances in Metal-Catalyzed 1,2-Difunctionalization of Conjugated Dienes
Received date: 2017-04-18
Revised date: 2017-05-22
Online published: 2017-05-25
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21232004, 21672142), the Program of Shanghai Subject Chief Scientists (No. 14XD1402300) and the Basic Research Foundation of Shanghai Science and Technology Committee (No. 15JC1402200).
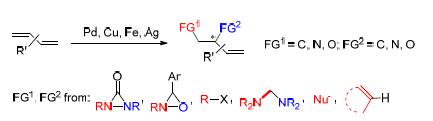
共轭二烯的1,2-双官能化反应是重要的均相催化反应之一.反应所得的双官能化产物广泛存在于天然产物和生物活性的化合物中,也是很多重要的有机中间体的来源;而且双官能化产物中还保留有一个可以继续转化的双键,可以更灵活地构建我们所需的目标结构,或继续官能化,从而实现简单二烯的多官能化反应,得到邻近的多官能化产物.此领域中主要的难点在于反应中区域选择性、化学选择性和立体选择性等复杂选择性的控制.近年来,随着金属有机化学的不断发展,已陆续实现了金属钯、铜、铁或银催化的共轭二烯的1,2-双官能化反应,而且有些报道中通过引入手性配体成功地实现了共轭二烯的不对称1,2-双官能化反应.本综述将对近年来金属催化的共轭二烯的1,2-双官能化反应的研究进展进行重点介绍.
吴正兴 , 张万斌 . 金属催化的共轭二烯的1,2-双官能化反应的研究进展[J]. 有机化学, 2017 , 37(9) : 2250 -2262 . DOI: 10.6023/cjoc201704031
The 1,2-difunctionalization of conjugated dienes is an important homogeneous catalytic reaction. The obtained products through 1,2-difunctionalization are widely existed in natural products and bioactive compounds, and are also sources of important organic intermediates, in addition, the preserved double bond in the difunctionalized product can be further transformed to give the desired structures or be functionalized sequentially to achieve multi-functionalization. The main difficulties focus on the encountered complex selectivities, including the regioselectivity, chemoselectivity and stereoselectivity in reactions. In recent years, with the development of organometallic chemistry, metal palladium, copper, iron or silver catalyzed 1,2-difunctionalizations of conjugated dienes have been reported in succession. In some cases the enantioselective 1,2-difunctionalizations of conjugated dienes were achieved via the introduction of chiral ligands. This review mainly focus on the recent metal catalyzed 1,2-difunctionalizations of conjugated dienes.

Key words: metal-catalyzed; conjugated diene; 1,2-difunctionalization
[1] (a) Kolb, H. C.; VanNieuwenhze, M. S.; Sharpless, K. B. Chem. Rev. 1994, 94, 2483.
(b) Bäckvall, J.-E. Modern Oxidation Methods, Wiley-VCH, Weinheim, 2004.
(c) Kotov, V.; Scarborough, C. C.; Stahl, S. S. Inorg. Chem. 2007, 46, 1910.
(d) Minatti, A.; Muñiz, K. Chem. Soc. Rev. 2007, 36, 1142.
(e) Chemler, S. R.; Fuller, P. H. Chem. Soc. Rev. 2007, 36, 1153.
(f) Jensen, K. H.; Sigman, M. S. Org. Biomol. Chem. 2008, 6, 4083.
(g) McDonald, R. I.; Liu, G.; Stahl, S. S. Chem. Rev. 2011, 111, 2981.
(h) Niu, F.; Nie, C.; Chen, Y.; Sun, X. Prog. Chem. 2014, 26, 1942(in Chinese). (牛凡凡, 聂昌军, 陈勇, 孙小玲, 化学进展, 2014, 26, 1942.)
(i) He, T.; Zeng, X. Chin. J. Org. Chem. 2017, 37, 798(in Chinese). (何天雄, 曾祥华, 有机化学, 2017, 37, 798.)
[2] (a) Smith, J. G. Synthesis 1984, 629.
(b) Tanner, D. Angew. Chem. Int. Ed. 1994, 33, 599.
(c) Kolb, H. C.; VanNieuwenhze, M. S.; Sharpless, K. B. Chem. Rev. 1994, 94, 2483.
(d) Ager, D. J.; Prakash, I.; Schaad, D. R. Chem. Rev. 1996, 96, 835.
(e) Bennani, Y. L.; Hanessian, S. Chem. Rev. 1997, 97, 3161.
(f) Lucet, D.; Gall, T. L.; Mioskowski, C. Angew. Chem. Int. Ed. 1998, 37, 2580.
(g) Gribble, G. W. Acc. Chem. Res., 1998, 31, 141.
(h) Bergmeier, S. C. Tetrahedron 2000, 56, 2561.
(i) Lauret, C. Tetrahedron:Asymmetry 2001, 12, 2359.
(j) Schneider, C. Synthesis 2006, 3919.
(k) Singh, G. S.; D'hooghe, M.; Kimpe, N. D. Chem. Rev. 2007, 107, 2080.
(l) Gao, Z.; Xiao, L.; Chen, J.; Xia, C. Chin. J. Catal. 2008, 29, 831(in Chinese). (高志文, 肖林飞, 陈静, 夏春谷, 催化学报, 2008, 29, 831.)
(m) Bataille, C. J. R.; Donohoe, T. J. Chem. Soc. Rev. 2011, 40, 114.
(n) Callebaut, G.; Meiresonne, T.; Kimpe, N. D.; Mangelinckx, S. Chem. Rev. 2014, 114, 7954.
(o) Wang, Q.; Chang, H.; Wei, W.; Liu, Q.; Gao, W.; Li, Y.; Li, X. Chin. J. Org. Chem. 2016, 36, 939(in Chinese). (王清宇, 常宏宏, 魏文珑, 刘强, 高文超, 李彦威, 李兴, 有机化学, 2016, 36, 939.)
[3] Prileschajew, N. Eur. J. Inorg. Chem. 1909, 42, 4811.
[4] Gilman, H. Organic Chemistry:An Advanced Treatise, Vol. 1, Wiley, New York, 1938, p. 36.
[5] (a) Criegee, R. Justus Liebigs Ann. Chem. 1936, 522, 75.
(b) Criegee, R. Angew. Chem. 1937, 50, 153.
[6] Kwart, H.; Kahn, A. A. J. Am. Chem. Soc. 1967, 89, 1950.
[7] (a) Sharpless, K. B.; Patrick, D. W.; Truesdale, L. K.; Biller, S. A. J. Am. Chem. Soc. 1975, 97, 2305.
(b) Chong, A. O.; Oshima, K.; Sharpless, K. B. J. Am. Chem. Soc. 1977, 99, 3420.
[8] (a) Bäckvall, J.-E. Tetrahedron Lett. 1978, 19, 163.
(b) Bäckvall, J.-E.; Björkman, E. E. J. Org. Chem. 1980, 45, 2893.
[9] (a) Sharpless, K. B.; Chong, A. O.; Oshima, K. J. Org. Chem. 1976, 41, 177.
(b) Hentges, S. G.; Sharpless, K. B. J. Am. Chem. Soc. 1980, 102, 4263.
(c) Katsuki, T.; Sharpless, K. B. J. Am. Chem. Soc. 1980, 102, 5974.
(d) Jacobsen, E. N.; Marko, I.; Mungall, W. S.; Schroeder, G.; Sharpless, K. B. J. Am. Chem. Soc. 1988, 110, 1968.
[10] (a) Aranyos, A.; Szabó, K. J.; Bäckvall, J.-E. J. Org. Chem. 1998, 63, 2523.
(b) Itami, K.; Palmgren, A.; Thorarensen, A.; Bäckvall, J.-E. J. Org. Chem. 1998, 63, 6466.
(c) Palmgren, A.; Larsson, A. L. E.; Bäckvall, J.-E. J. Org. Chem. 1999, 64, 836.
(d) Löfstedt, J.; Närhi, K.; Dorange, I.; Bäckvall, J.-E. J. Org. Chem. 2003, 68, 7243.
(e) Verboom, R. C.; Persson, B. A.; Bäckvall, J.-E. J. Org. Chem. 2004, 69, 3102.
(f) Piera, J.; Persson, A.; Caldentey, X.; Bäckvall, J.-E. J. Am. Chem. Soc. 2007, 129, 14120.
(g) Burks, H. E.; Kliman, L. T.; Morken, J. P. J. Am. Chem. Soc. 2009, 131, 9134.
(h) Schuster, C. H.; Li, B.; Morken, J. P. Angew. Chem. Int. Ed. 2011, 50, 7906.
[11] Xu, D.; Crispino, G. A.; Sharpless, K. B. J. Am. Chem. Soc. 1992, 114, 7571.
[12] O'Connor, J. M.; Stallman, B. J.; Clark, W. G.; Shu, A. Y. L.; Spada, R. E.; Stevenson, T. M.; Dieck, H. A. J. Org. Chem. 1983, 48, 807.
[13] (a) Larock, R. C.; Fried, C. A. J. Am. Chem. Soc. 1990, 112, 5882.
(b) Larock, R. C.; Berrios-Pena, N. G.; Narayanan, K. J. Org. Chem. 1990, 55, 3447.
[14] (a) Du, H.; Zhao, B.; Shi, Y. J. Am. Chem. Soc. 2007, 129, 762.
(b) Zhao, B.; Du, H.; Cui, S.; Shi, Y. J. Am. Chem. Soc. 2010, 132, 3523.
[15] Liao, L.; Jana, R.; Urkalan, K. B.; Sigman, M. S. J. Am. Chem. Soc. 2011, 133, 5784.
[16] Larock, R. C.; Harrison, L. W.; Hsu, M. H. J. Org. Chem. 1984, 49, 3662.
[17] Bar, G. L. J.; Lloyd-Jones, G. C.; Booker-Milburn, K. I. J. Am. Chem. Soc. 2005, 127, 7308.
[18] Houlden, C. E.; Bailey, C. D.; Ford, J. G.; Gagné, M. R.; Lloyd-Jones, G. C.; Booker-Milburn, K. I. J. Am. Chem. Soc. 2008, 130, 10066.
[19] Xing, D.; Yang, D. Org. Lett. 2013, 15, 4370.
[20] Cooper, S. P.; Booker-Milburn, K. I. Angew. Chem. Int. Ed. 2015, 54, 6496.
[21] (a) Kagechika, K.; Shibasaki, M. J. Org. Chem. 1991, 56, 4093.
(b) Kagechika, K.; Ohshima, T.; Shibasaki, M. Tetrahedron 1993, 49, 1773.
(c) Ohshima, T.; Kagechika, K.; Adachi, A.; Sodeoka, M.; Shibasaki, M. J. Am. Chem. Soc. 1996, 118, 7108.
[22] Du, H.; Yuan, W.; Zhao, B.; Shi, Y. J. Am. Chem. Soc. 2007, 129, 11688.
[23] Cornwall, R. G.; Zhao, B.; Shi, Y. Org. Lett. 2013, 15, 796.
[24] Stokes, B. J.; Liao, L.; de Andrade, A. M.; Wang, Q.; Sigman, M. S. Org. Lett. 2014, 16, 4666.
[25] Wu, X.; Lin, H.-C.; Li, M.-L.; Li, L. L.; Han, Z.-Y.; Gong, L.-Z. J. Am. Chem. Soc. 2015, 137, 13476.
[26] Liu, Y.; Xie, Y.; Wang, H.; Huang, H. J. Am. Chem. Soc. 2016, 138, 4314.
[27] Chen, S.-S.; Meng, J.; Li, Y.-H.; Han, Z.-Y. J. Org. Chem., 2016, 81, 9402.
[28] Chen, S.-S.; Wu, M.-S.; Han, Z.-Y. Angew. Chem., Int. Ed. 2017, 56, 6641.
[29] Yuan, W.; Du, H.; Zhao, B.; Shi, Y. Org. Lett. 2007, 9, 2589.
[30] Zhao, B.; Peng, X.; Cui, S.; Shi, Y. J. Am. Chem. Soc. 2010, 132, 11009.
[31] Zhao, B.; Peng, X.; Zhu, Y.; Ramirez, T. A.; Cornwall, R. G.; Shi, Y. J. Am. Chem. Soc. 2011, 133, 20890.
[32] Michaelis, D. J.; Ischay, M. A.; Yoon, T. P. J. Am. Chem. Soc. 2008, 130, 6610.
[33] Du, H.; Zhao, B.; Yuan, W.; Shi, Y. Org. Lett. 2008, 10, 4231.
[34] Zhao, B.; Du, H.; Shi, Y. J. Org. Chem. 2009, 74, 8392.
[35] Williamson, K. S.; Yoon, T. P. J. Am. Chem. Soc. 2010, 132, 4570.
[36] Williamson, K. S.; Yoon, T. P. J. Am. Chem. Soc. 2012, 134, 12370.
[37] (a) Llaveria, J.; Beltrán, Á.; Díaz-Requejo, M. M.; Matheu, M. I.; Castillón, S.; Pérez, P. J. Angew. Chem. Int. Ed. 2010, 49, 7092.
(b) Llaveria, J.; Beltrán, Á.; Sameera, W. M. C.; Locati, A.; Díaz-Requejo, M. M.; Matheu, M. I.; Castillón, S.; Maseras, F.; Pérez, P. J. J. Am. Chem. Soc. 2014, 136, 5342.
/
| 〈 |
|
〉 |