

含能化合物3,3'-双(氟二硝甲基-ONN-氧化偶氮基)氧化偶氮呋咱合成与表征
收稿日期: 2017-01-05
修回日期: 2017-03-14
网络出版日期: 2017-06-02
基金资助
国家自然科学基金(No.21243007)资助项目.
Synthesis and Characterization of an Energetic Compound 3,3'-Bis(fluoronitromethyl-ONN-azoxy) azoxyfurazan
Received date: 2017-01-05
Revised date: 2017-03-14
Online published: 2017-06-02
Supported by
Project supported by the National Natural Science Foundation of China (No.21243007).
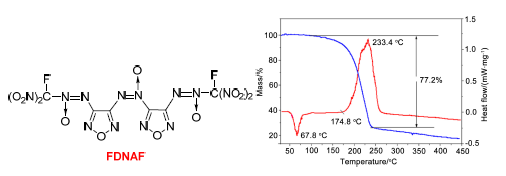
以二氨基氧化偶氮呋咱(DAOAF)和2,2-二甲基-5-硝基-5-亚硝基-1,3-二氧环己烷(DMNNDO)为原料,通过氧化偶联、水解、溴化、还原、硝化、成盐、氟化等7步反应,合成了含能化合物3,3'-双(氟二硝甲基-ONN-氧化偶氮基)氧化偶氮呋咱(FDNAF),并采用IR、1H NMR、13C NMR、15N NMR、19F NMR和元素分析等手段对中间体及目标化合物进行了结构表征.改进了水解反应条件,采用HCl/CH3CH2OH代替AcCl/CH3OH水解体系,反应时间由18 h缩短为2 h,粗品纯度提高至93%.研究了溴化反应的最佳条件,在20℃反应30 min获得溴化产物,收率最高为51.5%.基于量子化学密度泛函理的B3LYP方法,对FDNAF的13C NMR,15N NMR的化学位移和IR吸收频率进行理论研究,实验结果和理论结果具有较好的一致性.开展了单硝基甲基-ONN-氧化偶氮化合物(BNMAF)、二硝基甲基-ONN-氧化偶氮化合物(BDNAF)和氟二硝甲基-ONN-氧化偶氮化合物(FDNAF)三种含能化合物的物化、爆轰性能和热性能研究,含能化合物FDNAF理论密度2.02 g·cm-3、热分解峰温233.4℃、氧平衡6.72%、爆速9735 m·s-1、爆压44.9 GPa、特性落高36 cm,表明FDNAF是一种性能突出的高能量密度含能化合物.
关键词: 有机化学; 氟二硝甲基-ONN-氧化偶氮基; 含能化合物; 合成
张家荣 , 毕福强 , 廉鹏 , 张俊林 , 王伯周 . 含能化合物3,3'-双(氟二硝甲基-ONN-氧化偶氮基)氧化偶氮呋咱合成与表征[J]. 有机化学, 2017 , 37(10) : 2736 -2744 . DOI: 10.6023/cjoc201701014
Using 3,3'-diamino-4,4'-azoxyfurazan (DAOAF) and 2,2-dimethyl-5-nitro-5-nitroso-1,3-dioxane (DMNNDO) as starting materials, energetic compound 3,3'-bis(fluoronitromethyl-ONN-azoxy)azoxyfurazan (FDNAF) was designed and synthesized via oxidation coupling, hydrolysis, bromization, reduction, nitration, salification and fluorination etc., and the structures of all the intermediates and the title compound were characterized by IR, 1H NMR, 13C NMR, 15N NMR、19F NMR and elemental analysis. Using HCl/CH3CH2OH as the hydrolysis system instead of AcCl/CH3OH, the condition of hydrolysis reaction was optimized, the reaction time was shortened from 18 h to 2 h and the purity of raw product was improved to 93%. The bromination reaction conditions were also studied. Under the optimum conditions with the temperature of 20℃ and the reaction time of 30 min, the brominated product was obtained with a yield of 51.5%. Based on B3LYP method of density function theory, 13C NMR, 15N NMR and IR chemical shifts were studied theoretically, which agreed with experimental data. The physicochemical properties, detonation performances and thermal behaviors of 3,3'-bis(nitromethyl-ONN-azoxy)azoxyfurazan (BNMAF), 3,3'-bis(dinitromethyl-ONN-azoxy)azoxyfurazan (BDNAF) and FDNAF were studied and analyzed. The results proved that FDNAF is a potential energetic compound with the theoretical density of 2.02 g·cm-3, the decomposition point of 233.4℃, the oxygen balance of 6.72%, the explosion velocity of 9735 m·s-1, the detonation pressure of 44.9 GPa, and the characteristic drop height of impact sensitivity of 36 cm.

[1] Zhang, J.-L.; Xiao, C.; Zhai, L.-J.; Wang, X.-J.; Bi, F.-Q.; Wang, B.-Z. Chin. J. Org. Chem. 2016, 36, 1197(in Chinese). (张俊林, 肖川, 翟连杰, 王锡杰, 毕福强, 王伯周, 有机化学, 2016, 36, 1197.)
[2] Qiu, L.; Xu, X.-J.; Xiao, H.-M. Chin. J. Energ. Mater. 2005, 13, 262(in Chinese). (邱玲, 许晓娟, 肖鹤鸣, 含能材料, 2005, 13, 262.)
[3] Pang, S.-P.; Sheng, F.-F.; Lv, H.-F.; Dong, K.; Zhang, Y.-Y.; Sun, C.-H.; Song, J.-W.; Zhao, X.-Q. Acta Armamentarii 2014, 5, 725(in Chinese). (庞思平, 申帆帆, 吕芃浩, 董凯, 张义迎, 孙成辉, 宋建伟, 赵信歧, 兵工学报, 2014, 5, 725.)
[4] Zhai, L.-J.; Wang, B.-Z.; Huo, H.; Li, H.; Li, Y.-N.; Huang, X.-P.; Liu, N.; Fan, X.-Z. Chin. J. Org. Chem. 2013, 33, 1755(in Chinese). (翟连杰, 王伯周, 霍欢, 李辉, 黄新萍, 刘宁, 樊学忠, 有机化学, 2013, 33, 1755.)
[5] Tang, Y.; Gao, H.; Parrish, D. A.; Shreeve, J. M. Chemistry (Weinheim an der Bergstrasse, Germany) 2015, 21(32), 11401.
[6] Xu, C.; Bi, F. Q.; Zhang, M.; Li, Q.; Ding, K. W.; Ge, Z. X. Chin. J. Struct. Chem. 2015, 34, 1341.
[7] Zhai, L. J.; Wang, B. Z.; Fan, X. Z.; Li, X. Z. Chin. J. Struct. Chem. 2014, 33, 1353.
[8] Luk'yanov, O. A.; Pokhvisneva, G. V.; Ternikova, T. V. Russ. Chem. Bull. 2012, 61, 1783.
[9] Luk'yanov, O. A.; Salamonov, Y. B.; Struchkov, Y. T.; Burtsev, Y. N.; Viadimir, S. K. Mendeleev Commun. 1992, 2, 52.
[10] Luk'yanov, O. A.; Pokhvisneva, G. V.; Ternikova, T. V.; Shlykova, N. I.; Shagaeva, M. E. Russ. Chem. Bull. 2011, 60, 1703.
[11] Luk'yanov, O. A.; Pokhvisneva, G. V.; Ternikova, T. V.; Shlykova, N. I. Russ. Chem. Bull. 2012, 61, 360.
[12] Luk'yanov, O. A.; Parakhin, V. V.; Pokhvisneva, G. V.; Ternikova, T. V. Russ. Chem. Bull. 2012, 61, 355.
[13] Luk'yanov, O. A.; Parakhin, V. V. Russ. Chem. Bull. 2012, 61, 1582.
[14] Gottardi, W. Monatsh. Chem. 1968, 99, 815.
[15] Luk'yanov, O. A.; Pokhvisneva, G. V.; Ternikova, T. V. Russ. Chem. Bull. 2015, 64, 137.
[16] Luk'yanov, O. A.; Salamonov, B. Y.; Bass, G. A.; Strelenko, Y. Bull. Acad. Sci. USSR, Div. Chem. Sci. 1991, 40, 93.
[17] Luk'yanov, O. A.; Salamonov, B. Y.; Bass, G. A. Izv. Akad. Nauk, Ser. Khim. 1992, 10, 2400.
[18] Luk'yanov, O. A.; Pokhvisneva, G. V.; Ternikova, T. V. Russian 2581050, 2016.
[19] Li, H.-Z.; Zhou, X.-Q.; Li, J.-S.; Huang, M. Chin. J. Org. Chem. 2008, 28, 1646(in Chinese). (李洪珍, 周小清, 李金山, 黄明, 有机化学, 2008, 28, 1646.)
[20] Zhou, Y.-S.; Zhou, C.; Wang, B.-Z.; Li, J.-K.; H. H.; Zhang, Y.-G.; Wang, X.-J.; Luo, Y.-F. Chin. J. Energ. Mater. 2011, 19, 509(in Chinese). (周彦水, 周诚, 王伯周, 李健康, 霍欢, 张叶高, 王锡杰, 罗义芬, 含能材料, 2011, 19, 509.)
[21] Li, X.-Z.; Wang, B.-Z.; Li, H.; Li, Y.-N.; Bi, F.-Q.; Fan, X.-Z. Chin. J. Org. Chem. 2012, 32, 1975(in Chinese). (李祥志, 王伯周, 李辉, 李亚南, 毕福强, 樊学忠, 有机化学, 2012, 32, 1975.)
[22] Jia, S.-Y.; Zhang, H.-H.; Zhou, C.; Lai, W.-P.; Li, X.-Z.; Wang, B.-Z. Chin. J. Org. Chem. 2015, 35, 851(in Chinese). (贾思媛, 张海昊, 周诚, 来蔚鹏, 李祥志, 王伯周, 有机化学, 2015, 35, 851.)
[23] Bi, F.-Q.; Wang, Y.; Wang, B.-Z.; Zhang, J.-R.; Zhang, J.-L.; Zhai, L.-J.; Li, X.-Z. Chin. J. Energ. Chem. 2016, 35, 851(in Chi-nese). (毕福强, 王玉, 王伯周, 张家荣, 张俊林, 翟连杰, 李祥志, 含能材料, 2016, 35, 851.)
[24] Wang, M.-C.; Bi, F.-Q.; Zhang, G.; Luan, J.-Y.; Xu, M.; Ning, Y.-L.; Fan, X.-Z. Chin. J. Energ. Mater. 2013. 21, 473(in Chinese). (王民昌, 毕福强, 张皋, 栾洁玉, 徐敏, 宁艳利, 樊学忠, 含能材料, 2016, 21, 473.)
[25] Becke, A. D. J. Chem. Phys. 1993, 98, 5648.
[26] Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Dapprich, S., Gaussian 98, Revision A.7; Gaussian Inc., Pittsburgh, PA, 1998, 40.
[27] Wu, X.; Long, X.-P.; He, B. Sci. China, B 2008, 38, 1129(in Chinese). (吴雄, 龙新平, 何碧, 中国科学(B辑), 2008, 38, 1129.)
[28] Politzer, P.; Murray, J. S. Cent. Eur. J. Energy Mater. 2011, 8, 209.
[29] Karakaya, P.; Sidhoum, M.; Christodoulatos, C.; Nicolich, S.; Balas, W. Hazard. Mater. 2005, 120, 183.
[30] Pospísil, M.; Vavra, P.; Concha, M. C.; Murray, J. S.; Politzer, P. J. Mol. Model. 2010, 16, 895.
/
| 〈 |
|
〉 |