

钯-单膦催化剂在烯烃不对称硅氢化反应中的应用
收稿日期: 2017-04-10
修回日期: 2017-05-15
网络出版日期: 2017-06-07
基金资助
国家自然科学基金(No.21302051)和湖南省自然科学基金(No.14JJ3091)资助项目.
Application of Pd-Monodentate Phosphorus Catalysts in the Asymmetric Hydrosilylation Reactions of Alkenes
Received date: 2017-04-10
Revised date: 2017-05-15
Online published: 2017-06-07
Supported by
Project supported by the National Natural Science Foundation of China (No.21302051) and the Natural Science Foundation of Hunan Province (No.14JJ3091).
张凤 , 刘祥华 , 刘玮 , 邓国军 . 钯-单膦催化剂在烯烃不对称硅氢化反应中的应用[J]. 有机化学, 2017 , 37(10) : 2555 -2568 . DOI: 10.6023/cjoc201704011
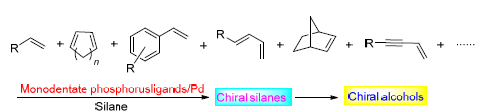
Asymmetric hydrosilylation of alkenes, which has been recognized as an important method for the preparations of optically active secondary alcohols, deserves widespread attention over the world. It is reported that such reaction can be catalyzed by Pd-monodentate phosphorus catalysts with excellent reactivity and enantioselectivity. In the past decades, a wide variety of chiral monodentate phosphorus ligands have been developed because of their stable structure, facile synthesis, convenient modification, unique efficiency. Among them, there are three predominant classes of ligands-phosphines based on an axially chiral biaryl scaffold, phosphines based on a planar chiral ferrocene scaffold and chiral phosphoramidites. Herein, the recent advances in asymmetric hydrosilylation of alkyl-substituted alkenes, styrene derivatives, 1,3-dienes and other carbon-carbon double bond compounds catalyzed by palladium monodentate phosphorus catalysts are summarized. The perspective is also discussed.
[1] (a) Kiss, G. Chem. Rev. 2001, 101, 3435.
(b) Liebscher, Y. J. Chem. Rev. 2007, 107, 133.
(c) Negishi, E. I.; Anastasia, L. Chem. Rev. 2003, 103, 1979.
(d) Ruiz-Castillo, P.; Buchwald, S. L. Chem. Rev. 2016, 116, 12564.
[2] (a) Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147.
(b) Wu, X. F.; Neumann, H.; Beller, M. Chem. Rev. 2013, 113, 1.
(c) Cacchi, S.; Fabrizi, G. Chem. Rev. 2005, 105, 2873.
(d) Li, Z.; Duan, W. L. Chin. J. Org. Chem. 2016, 36, 1805(in Chinese). (李振, 段伟良, 有机化学, 2016, 36, 1805.)
(e) Li, J. X.; Zhang, Z. M.; Li, C. S.; Luo, W.; Yang, S. R. Chin. J. Org. Chem. 2015, 35, 2199(in Chinese). (李建晓, 张振明, 李春生, 罗维, 杨少容, 有机化学, 2015, 35, 2199.)
[3] (a) Zimmer, R.; Dinesh, C. U.; Nandanan, E.; Khan, F. A. Chem. Rev. 2000, 100, 3067.
(b) Tietze, L. F.; Ila, H.; Bell, H. P. Chem. Rev. 2004, 104, 3453.
[4] McDonald, R. I.; Liu, G. S.; Stahl, S. S. Chem. Rev. 2011, 111, 2981.
[5] (a) Brunner, H.; Becker, R.; Riepl, G. Organometallics 1984, 3, 1354.
(b) Nishiyama, H.; Yamaguchi, S.; Kondo, M.; Itoh, K. J. Org. Chem. 1992, 57, 4306.
[6] Kiso, Y.; Yamamoto, K.; Tamao, K.; Kumada, M. J. Am. Chem. Soc. 1972, 94, 4373.
[7] (a) Teichert, J. F.; Feringa, B. L. Angew. Chem., Int. Ed. 2010, 49, 2486.
(b) Zhang, Z.; Xie, F.; Yang, B.; Yu, H.; Zhang, W. Chin. J. Org. Chem. 2011, 31, 429(in Chinese). (张振锋, 谢芳, 杨波, 余焓, 张万斌, 有机化学, 2011, 31, 429.)
(c) Minnaard, A. J.; Feringa, B. L.; Lefort, L.; de Vries, J. G. Acc. Chem. Res. 2007, 40, 1267.
(d) de Vries, A. H. M.; de Vries, J. G. Platinum Metals Rev. 2006, 50, 54.
[8] (a) Feng, X. Q.; Duan, H. F. Chin. J. Org. Chem. 2015, 35, 259(in Chinese). (冯向青, 杜海峰, 有机化学, 2015, 35, 259.)
(b) Rong, J.; Ni, C. F.; Wang, Y. Z.; Kuang, C. W.; Gu, Y. C.; Hu, J. B. Acta Chim. Sinica 2017, 75, 105(in Chinese). (荣健, 倪传法, 王云泽, 匡翠文, 顾玉诚, 胡金波, 化学学报, 2017, 75, 105.)
(c) Park, H. S.; Kim, M. Y.; Ahn, H. J.; Han, J. W. Bull. Korean Chem. Soc. 2016, 37, 795.
(d) Ogasawara, M.; Area, S.; Watanabe, S.; Nakajima, K.; Takahashi, T. ACS Catal. 2016, 6, 1308.
[9] (a) Gibson, S. E.; Rudd, M. Adv. Synth. Catal. 2007, 349, 781.
(b) Han, J. W.; Hayashi, T. Tetrahedron:Asymmetry 2010, 21, 2193.
(c) Han, J. W.; Hayashi, T. Tetrahedron:Asymmetry 2014, 25, 479.
[10] Liu, Z.; He, X. Proc. Chem. 2006, 18, 1489(in Chinese). (刘振德, 何煦昌, 化学进展, 2006, 18, 1489.)
[11] Hayashi, T.; Tamao, K.; Katsuro, Y.; Nakae, I.; Kumada, M. Tetrahedron Lett. 1980, 21, 1871.
[12] Uozumi, Y.; Kitayama, K.; Hayashi, T. Tetrahedron:Asymmetry 1993, 4, 2419.
[13] Hayashi, T.; Kabeta, K. Tetrahedron Lett. 1985, 26, 3023.
[14] Hayashi, T.; Matsumoto, Y.; Morikawa, I.; Ito, Y. Tetrahedron:Asymmetry 1990, 1, 151.
[15] Pioda, G.; Togni, A. Tetrahedron:Asymmetry 1998, 9, 3903.
[16] Weber, I.; Jones, G. B. Tetrahedron Lett. 2001, 42, 6983.
[17] Gibson, S. E.; Rendell, J. T.; Rudd, M. Synthesis 2006, 3631.
[18] (a) Pedersen, H. L.; Johannsen, M. Chem. Commun. 1999, 2517.
(b) Pedersen, H. L.; Johannsen, M. J. Org. Chem. 2002, 67, 7982.
[19] Ohmura, H.; Matsuhash, H.; Tanaka, M.; Kuroboshi, M.; Hiyama, T.; Hatanaka, Y.; Goda, K. J. Organomet. Chem. 1995, 499, 167.
[20] Han, J. W.; Tokunaga, N.; Hayashi, T. Helv. Chim. Acta 2002, 85, 3848.
[21] Han, J. W.; Tokunaga, N.; Hayashi, T. J. Am. Chem. Soc. 2001, 123, 12915.
[22] Ogasawara, M.; Ito, A.; Yoshida, K.; Hayashi, T. Organometallics 2006, 25, 2715.
[23] (a) Noyori, R.; Takaya, H. Acc. Chem. Res. 1990, 23, 345.
(b) Berthod, M.; Mignani, G.; Woodward, G.; Lemaire, M. Chem. Rev. 2005, 105, 1801.
(c) Gao, A.; Ye, Q.; Yu, J.; Liu, W. Chin. J. Org. Chem. 2017, 37, 47(in Chinese). (高安丽, 叶青松, 余娟, 刘伟平, 有机化学, 2017, 37, 47.)
[24] Uozumi, Y.; Hayashi, T. J. Am. Chem. Soc. 1991, 113, 9887.
[25] Uozumi, Y.; Lee, S. Y.; Hayashi, T. Tetrahedron Lett. 1992, 33, 7185.
[26] Uozumi, Y.; Hayashi, T. Tetrahedron Lett. 1993, 34, 2335.
[27] Kitayama, K.; Uozumi, Y.; Hayashi, T. J. Chem. Soc., Chem. Commun. 1995, 1533.
[28] Hayashi, T.; Hirate, S.; Kitayama, K.; Tsuji, H.; Torii, A.; Uo-zumi, Y. J. Org. Chem. 2001, 66, 1441.
[29] Hayashi, T.; Niizuma, S.; Kamikawa, T.; Suzuki, N.; Uozumi, Y. J. Am. Chem. Soc. 1995, 117, 9101.
[30] Bringmann, G.; Wuzik, A.; Breuning, M.; Henschel, P.; Peters, K.; Peters, E. M. Tetrahedron:Asymmetry 1999, 10, 3025.
[31] Kitayama, K.; Tsuji, H.; Uozumi, Y.; Hayashi, T. Tetrahedron Lett. 1996, 37, 4169.
[32] Hayashi, T.; Han, J. W.; Takeda, A.; Tang, J.; Nohmi, K.; Mukaide, K.; Tsuji, H.; Uozumi, Y. Adv. Synth. Catal. 2001, 343, 279.
[33] Han, J. W.; Hayashi, T. Chem. Lett. 2001, 976.
[34] Han, J. W.; Hayashi, T. Tetrahedron:Asymmetry 2002, 13, 325.
[35] Dotta, P.; Kumar, P. G. A.; Pregosin, P. S.; Albinati, A.; Rizzato, S. Organometallics 2004, 23, 2295.
[36] Tschoerner, M.; Pregosin, P.; Albinati, A. Organometallics 1999, 18, 670.
[37] Gladiali, S.; Pulacchini, S.; Fabbri, D.; Manassero, M.; Sansoni, M. Tetrahedron:Asymmetry 1998, 9, 391.
[38] Yasuike, S.; Kawara, S.; Okajima, S.; Seki, H.; Yamaguchi, K.; Kurita, J. Tetrahedron Lett. 2004, 45, 9135.
[39] Ficks, A.; Martinez-Botella, I.; Stewart, B.; Harrington, R. W.; Clegg, W.; Higham, L. J. Chem. Commun. 2011, 47, 8274.
[40] Duclos, M. C.; Singjunla, Y.; Petit, C.; Favre-Réguillon, A.; Jeanneau, E.; Popowycz, F.; Métay, E.; Lemaire, M. Tetrahedron Lett. 2012, 53, 5984.
[41] Fer, M. J.; Cinqualbre, J.; Bortoluzzi, J.; Chesse, M.; Leroux, F. R.; Panossian, A. Eur. J. Org. Chem. 2016, 26, 4545.
[42] Kiso, Y.; Yamamoto, K.; Tamao, K.; Kumada, M. J. Organomet. Chem. 1981, 210, 9.
[43] (a) Marinetti, A. Tetrahedron Lett. 1994, 35, 5861.
(b) Marinetti, A.; Ricard, L. Organometallics 1994, 13, 3956.
[44] (a) Okada, T.; Morimoto, T.; Achiwa, K. Chem. Lett. 1990, 999.
(b) Sakuraba, S.; Okada, T.; Morimoto, T.; Achiwa, K. Chem. Pharm. Bull. 1995, 43, 927.
[45] Yamomoto, T.; Yamada, T.; Nagata, Y.; Suginome, M. J. Am. Chem. Soc. 2010, 132, 7899.
[46] Hulst, R.; de Vries, N. K.; Feringa, B. L. Tetrahedron:Asymmetry 1994, 5, 699.
[47] (a) deVries, A. H. M.; Meetsma, A.; Feringa, B. L. Angew. Chem. Int. Ed. 1996, 35, 2375.
(b) Feringa, B. L. Acc. Chem. Res. 2000, 33, 346.
(c) López, F.; Minnaard, A. J.; Feringa, B. L. Acc. Chem. Res. 2007, 40, 179.
[48] (a) Van den Berg, M.; Minnaard, A. J.; Schudd, E. P.; van Esch, J.; de Vries, A. H. M.; de Vries, J. G. J. Am. Chem. Soc. 2000, 122, 11539.
(b) Hu, A. G.; Fu, Y.; Xie, J. H.; Zhou, H.; Wang, L. X.; Zhou, Q. L. Angew. Chem., Int. Ed. 2002, 41, 2348.
(c) Hou, G. H.; Xie, J. H.; Yan, P. C.; Zhou, Q. L. J. Am. Chem. Soc. 2009, 131, 1366.
(d) Liu, Y.; Ding, K. L. J. Am. Chem. Soc. 2005, 127, 10488.
[49] (a) Lipowsky, G.; Miller, N.; Helmchen, G. Angew. Chem., Int. Ed. 2004, 43, 4595.
(b) Ohmura, T.; Hartwig, J. F. J. Am. Chem. Soc. 2002, 124, 15164.
(c) Kiener, C. A.; Shu, C.; Incarvito, C.; Hartwig, J. F. J. Am. Chem. Soc. 2003, 125, 14272.
[50] Jensen, J. F.; Svendsen, B. Y.; la Cour, T. V.; Pedersen, H. L.; Johannsen, M. J. Am. Chem. Soc. 2002, 124, 4558.
[51] Guo, X. X.; Xie, J. H.; Hou, G. H.; Shi, W. J.; Wang, L. X.; Zhou, Q. L. Tetrahedron:Asymmetry 2004, 15, 2231.
[52] Li, X. S.; Song, J. A.; Xu, D. S.; Kong, L. C. Synthesis 2008, 925.
[53] Zhang, F.; Li, Y.; Li, Z. W.; He, Y. M.; Zhu, S. F.; Fan, Q. H.; Zhou, Q. L. Chem. Commun. 2008, 6048.
[54] Zhang, F.; Fan, Q. H. Org. Biomol. Chem. 2009, 7, 4470.
[55] Junge, K.; Wendt, B.; Enthaler, S.; Beller, M. ChemCatChem 2010, 2, 453.
[56] Park, H. S.; Namgung, S.; Shin, H. M.; Ahn, H. J.; Han, J. W. Bull. Korean Chem. Soc. 2014, 35, 2243.
[57] Park, H. S.; Han, J. W.; Shintani, R.; Hayashi, T. Tetrahedron:Asymmetry 2013, 24, 418.
[58] Park, H. S.; Shin, H. M.; Namgung, S.; Han, J. W. Bull. Korean Chem. Soc. 2014, 35, 2613.
[59] Ficks, A.; Hiney, R. M.; Harrington, R. W.; Gilheany, D. G.; Higham, L. J. Dalton Trans. 2012, 41, 3515.
[60] Fleming, J. T.; Ficks, A.; Waddell, P. G.; Paul, G.; Harrington, R. W.; Higham, L. J. Dalton Trans. 2016, 45, 1886.
[61] Tamura, M.; Fujihara, H. J. Am. Chem. Soc. 2003, 125, 15742.
[62] (a) Zhong, M. M.; Zhang, X. M.; Zhao, Y. P.; Li, C.; Yang, Q. H. Green Chem. 2015, 17, 1702.
(b) Shi, L.; Wang, X. W.; Sandoval, C. A.; Li, M. X.; Qi, Q. Y.; Li, Z. T.; Ding, K. L. Angew. Chem., Int. Ed. 2006, 45, 4108.
[63] (a) Xu, D. P.; Xiao, W. J.; Peng, J. J.; Li, J. Y.; Bai, Y. Chin. J. Org. Chem. 2014, 34, 2195(in Chinese). (徐大鹏, 肖文军, 彭家建, 厉嘉云, 白赢, 有机化学, 2014, 34, 2195.)
(b) Chen, L. Z.; Peng, J. J.; Li, J. Y.; Bai, Y.; Qiu, H. Y.; Lai, G. Q. Chin. J. Org. Chem. 2008, 28, 761(in Chinese). (陈玲珍, 彭家建, 厉嘉云, 白赢, 邱化玉, 来国桥, 有机化学, 2008, 28, 761.)
(c) Liu, S.; Peng, J. J.; Li, J. Y.; Bai, Y.; Xiao, W. J.; Lai, G. Q. Chin. J. Org. Chem. 2012, 32, 1827(in Chinese). (刘帅, 彭家建, 厉嘉云, 白赢, 肖文军, 来国桥, 有机化学, 2012, 32, 1827.)
[64] (a) Liu, Y. Y.; Zhang, W. B. Chin. J. Org. Chem. 2016, 36, 2249(in Chinese). (刘媛媛, 张万斌, 有机化学, 2016, 36, 2249.)
(b) Li, T. T.; Yu, P.; Lin, J. S.; Zhi, Y. G.; Liu, X. Y. Chin. J. Chem. 2016, 34, 490.
(c) Yang, M. B.; Wang, W. L.; Liu, Y.; Feng, L. J.; Ju, X. X. Chin. J. Chem. 2014, 32, 833.
(d) Gribble, M. W.; Pirnot, M. T.; Bandar, J. S.; Liu, R. Y.; Buchwald, S. L. J. Am. Chem. Soc. 2017, 139, 2192.
[65] Yang, L.; Lu, W.; Zhou, W.; Zhang, F. Green Chem. 2016, 18, 2941
/
| 〈 |
|
〉 |